200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95

Solved Please help me solve the following questions below

Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources - Choi - 2009 - ChemSusChem - Wiley Online Library

Chapter 10 Stoichiometry and Process Calculations

Thermodynamics: Synopsis, PDF, Solvation
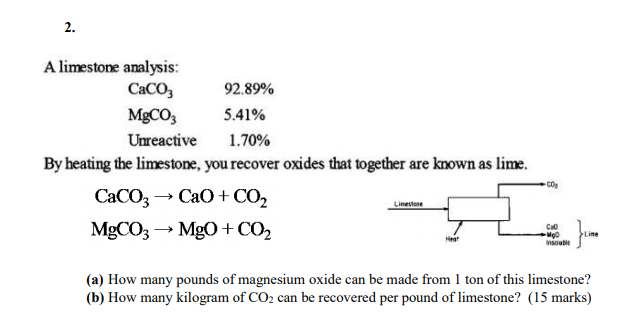
Solved A limestone analysis: CaCO, 92.89% MgCO3 5.41%


Copper Production - ScienceDirect
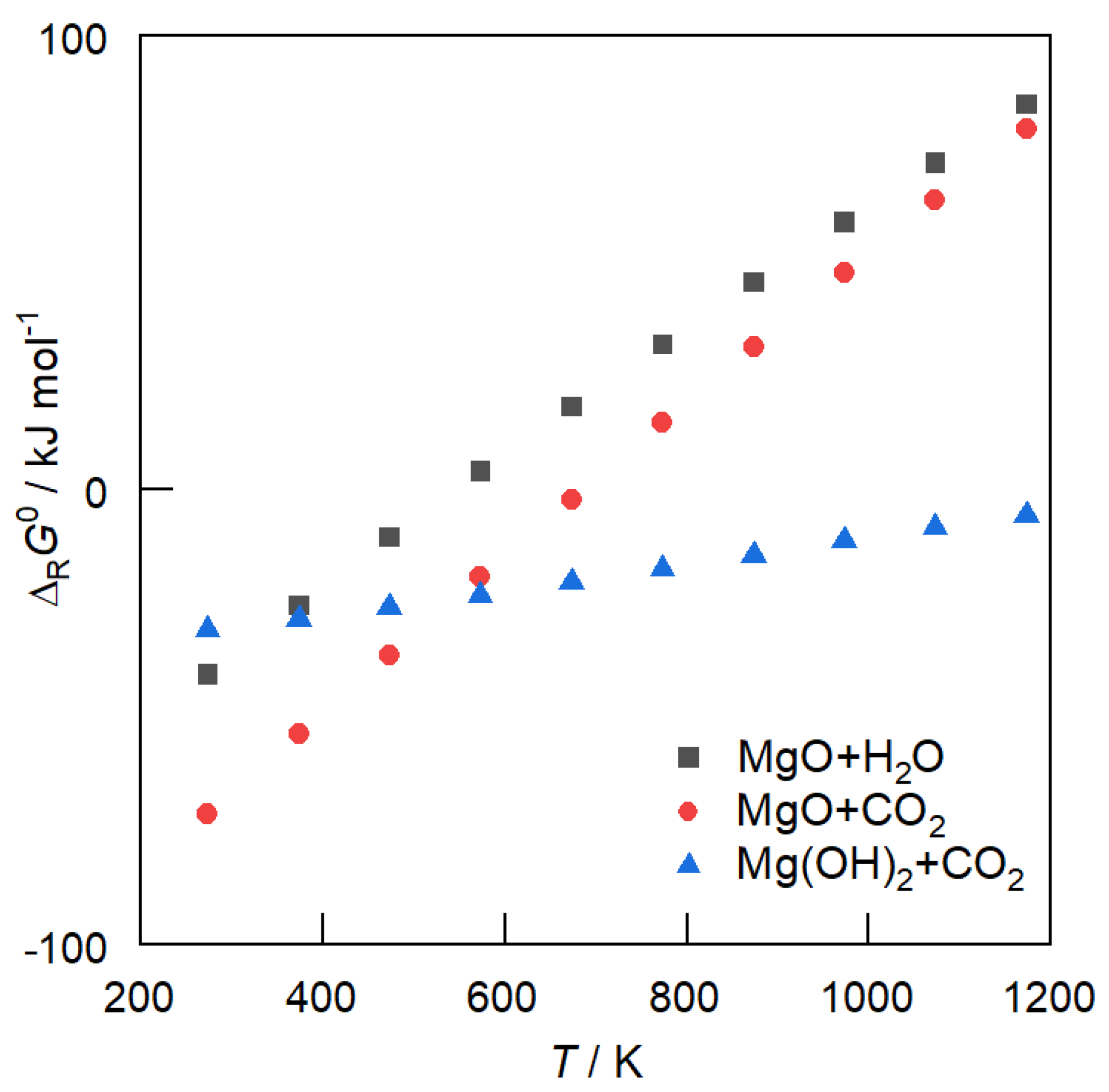
Energies, Free Full-Text

450 Question and answer Related to cement industry complete reference - INFINITY FOR CEMENT EQUIPMENT
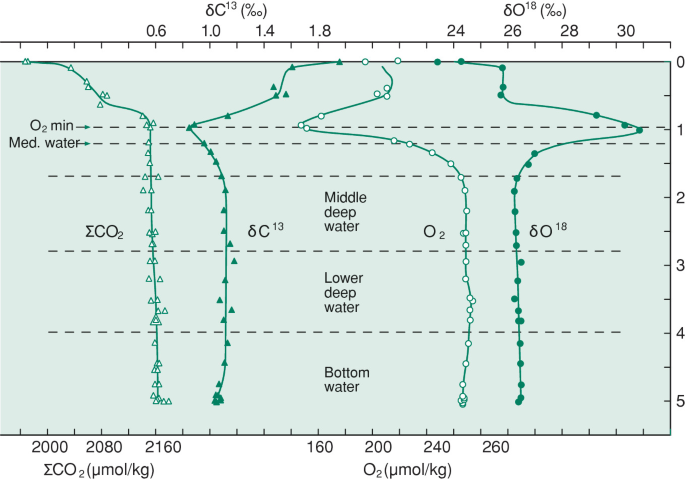
Variations of Stable Isotope Ratios in Nature

Amorphous-to-Crystalline Transition of Ca–Mg-Carbonates as a Function of Composition, Time, and Temperature
PDF) Measurements in Geochemical Carbon Dioxide Removal Citation for published version