The vapour pressure of a solution having 2.0 g of solute X (gram
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8
CH104: Chapter 7 - Solutions - Chemistry

Omguloporou Wass 22. The vapour pressure of a solution having 2.0 o of solute X (gram atomic mass = 32 g mol-') in 100 of CS, (vapour pressure = 854 torr) is

Chapter 16 Colligative properties of solutions

CHAPTER 12 PHYSICAL PROPERTIES OF SOLUTIONS

In ideal solution of non volatile solute B in solvent A in 2 : 5 molar ratio has vapour pressure 250

Understanding Liquid Solutions: Methods of Expressing Concentration, Vapour Pressure Concepts, and Raoult's Law, PDF, Solution

In ideal solution of non volatile solute B in solvent A in 2 : 5 molar ratio has vapour pressure 250

The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol-') in 100 g of CS, (vapour pressure = 854 torr) is 848.9 torr.
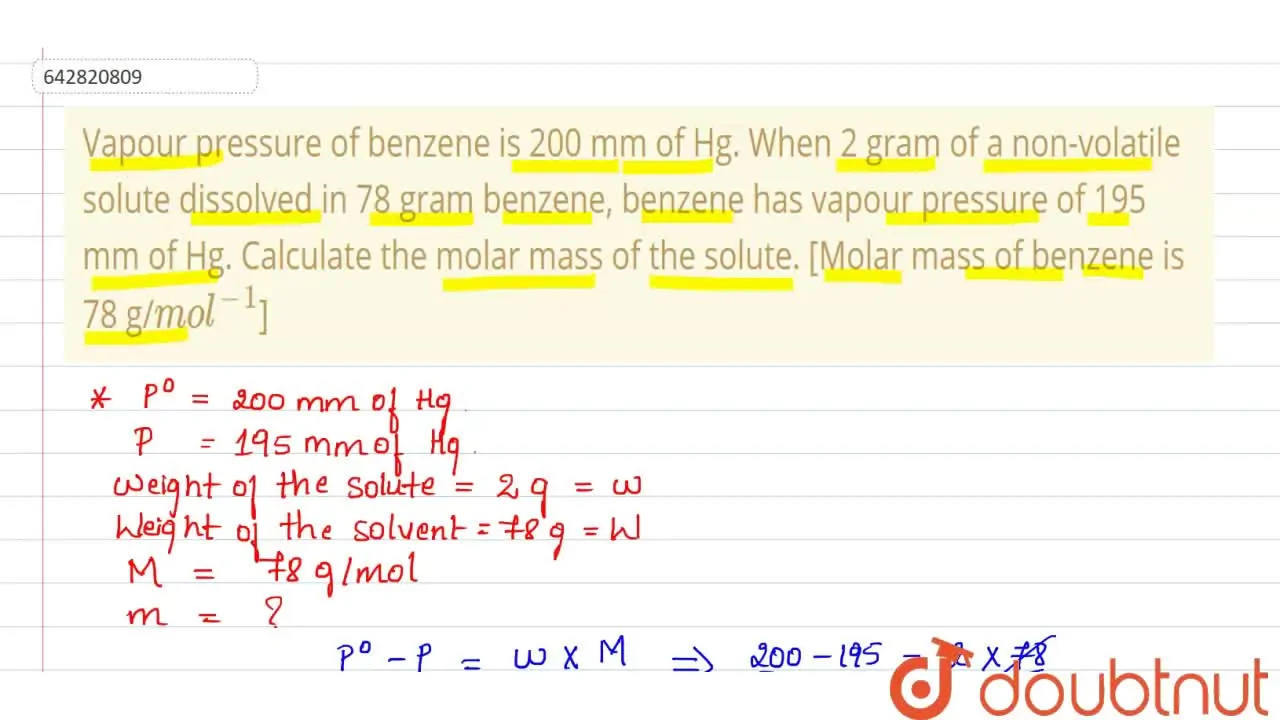
Kannada] Vapour pressure of benzene is 200 mm of Hg. When 2 gram of a

Chapter 2 Solutions