Breaking local symmetry—why water freezes but silica forms a glass

Everyone knows that water freezes at 0 degrees C. Life on Earth would be vastly different if this were not so. However, water
Everyone knows that water freezes at 0 degrees C. Life on Earth would be vastly different if this were not so. However, water's cousin, silica, exhibits wayward behavior when cooled that has long puzzled scientists.

The viscosities of several substances plotted as functions of the
U Tokyo – sciencesprings
Does water become less dense as it becomes colder or only when it reaches freezing temperature? - Quora

What causes that peak? Answering a long-standing question for covalent liquids

The shapes of water: New research details water's mysterious phase transitions
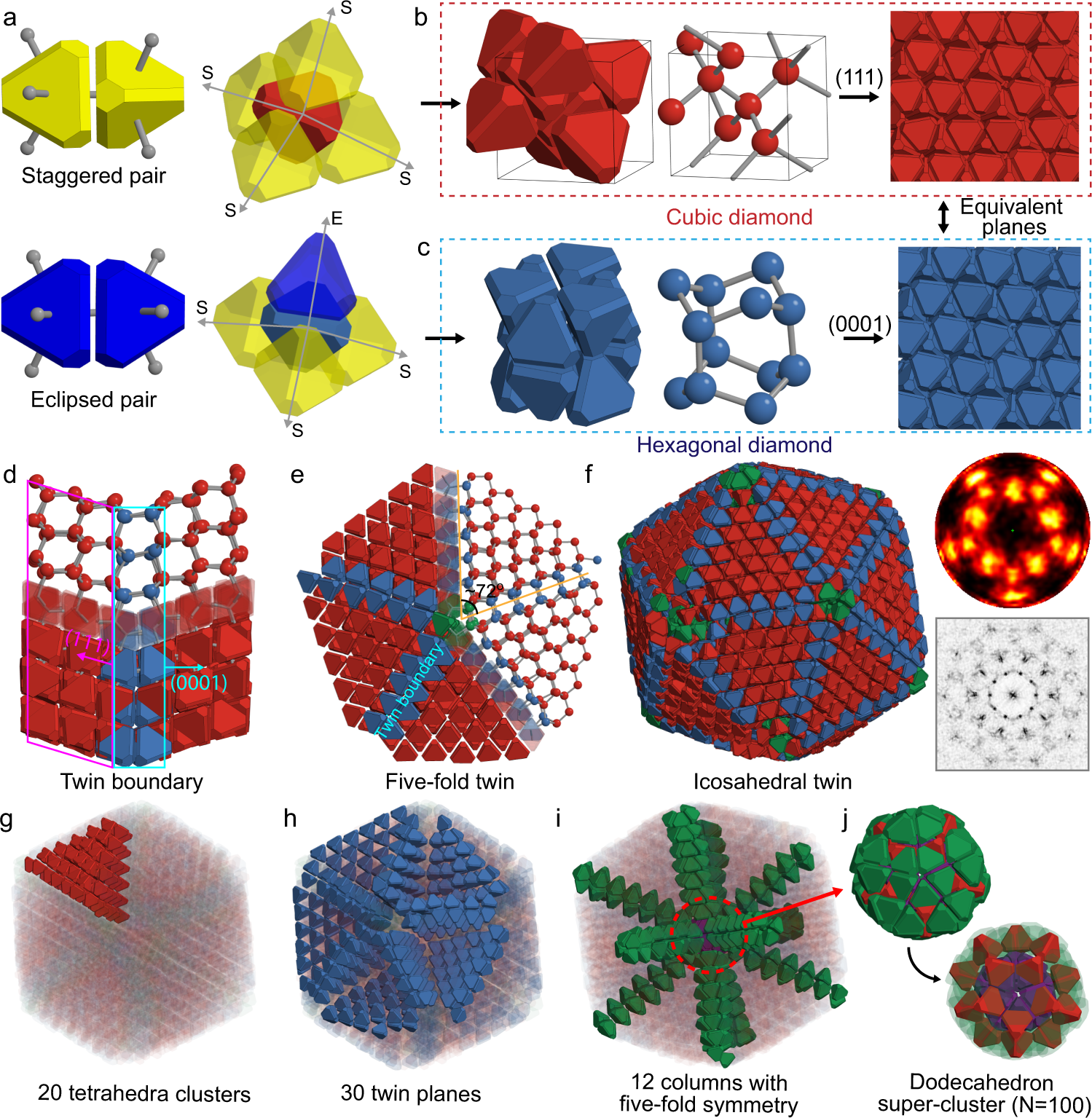
Entropically engineered formation of fivefold and icosahedral twinned clusters of colloidal shapes
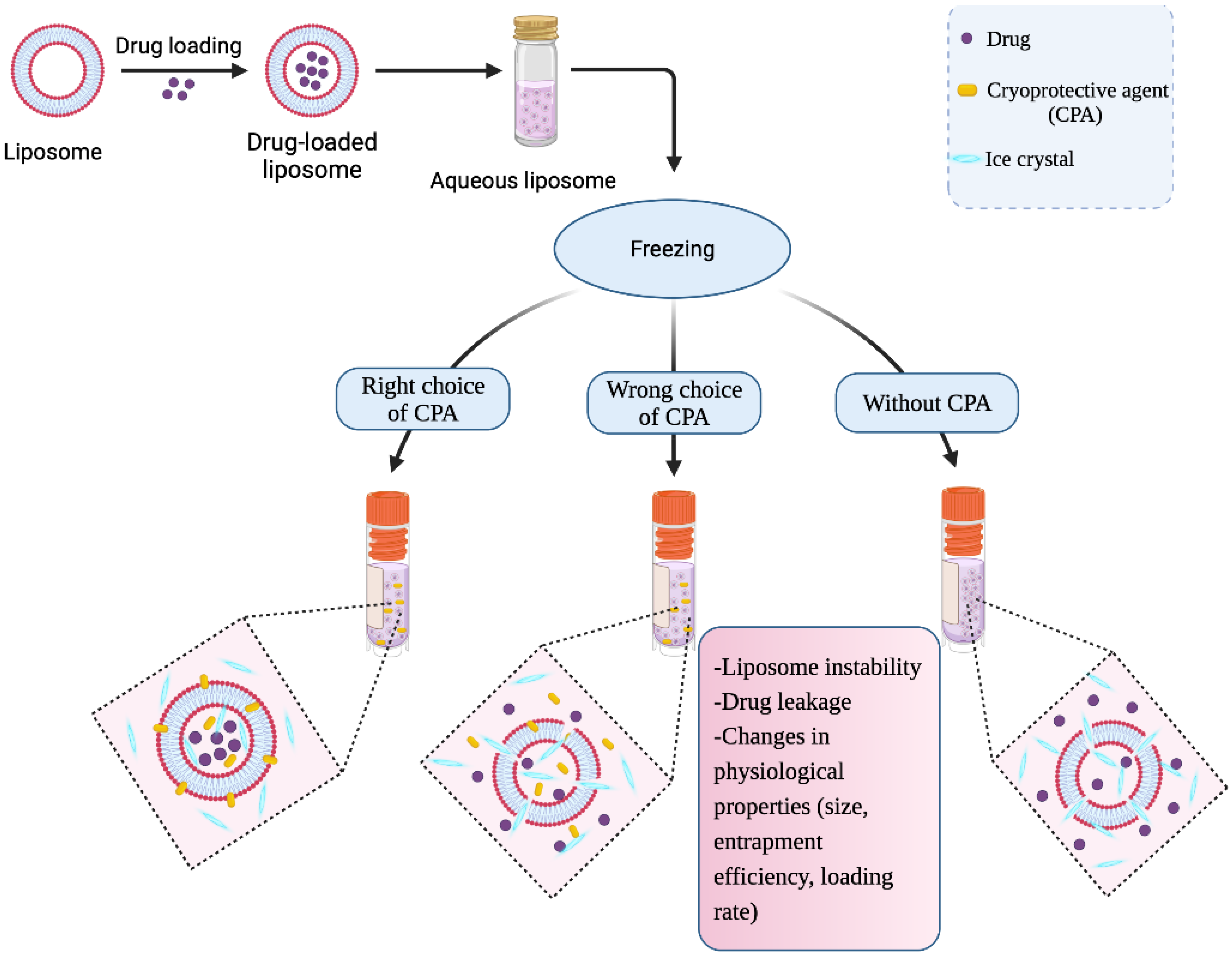
IJMS, Free Full-Text
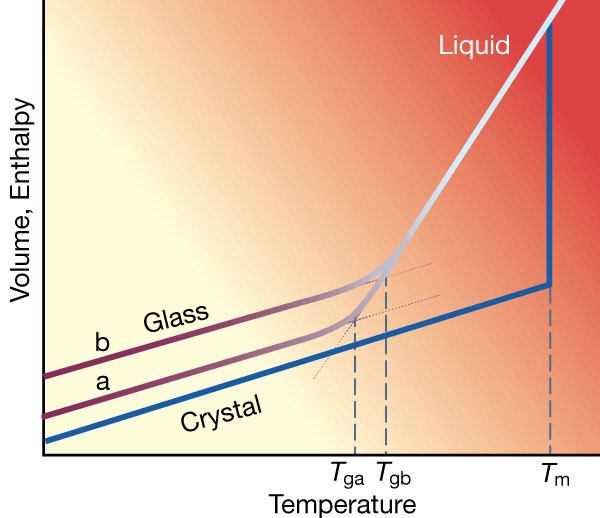
Supercooled liquids and the glass transition

Q&A: How Moving Water Freezes – SKY LIGHTS

Bendability of silicate glasses. a) Three‐point‐bending of a chemically

Recent progress in understanding the anti-icing behavior of materials - ScienceDirect
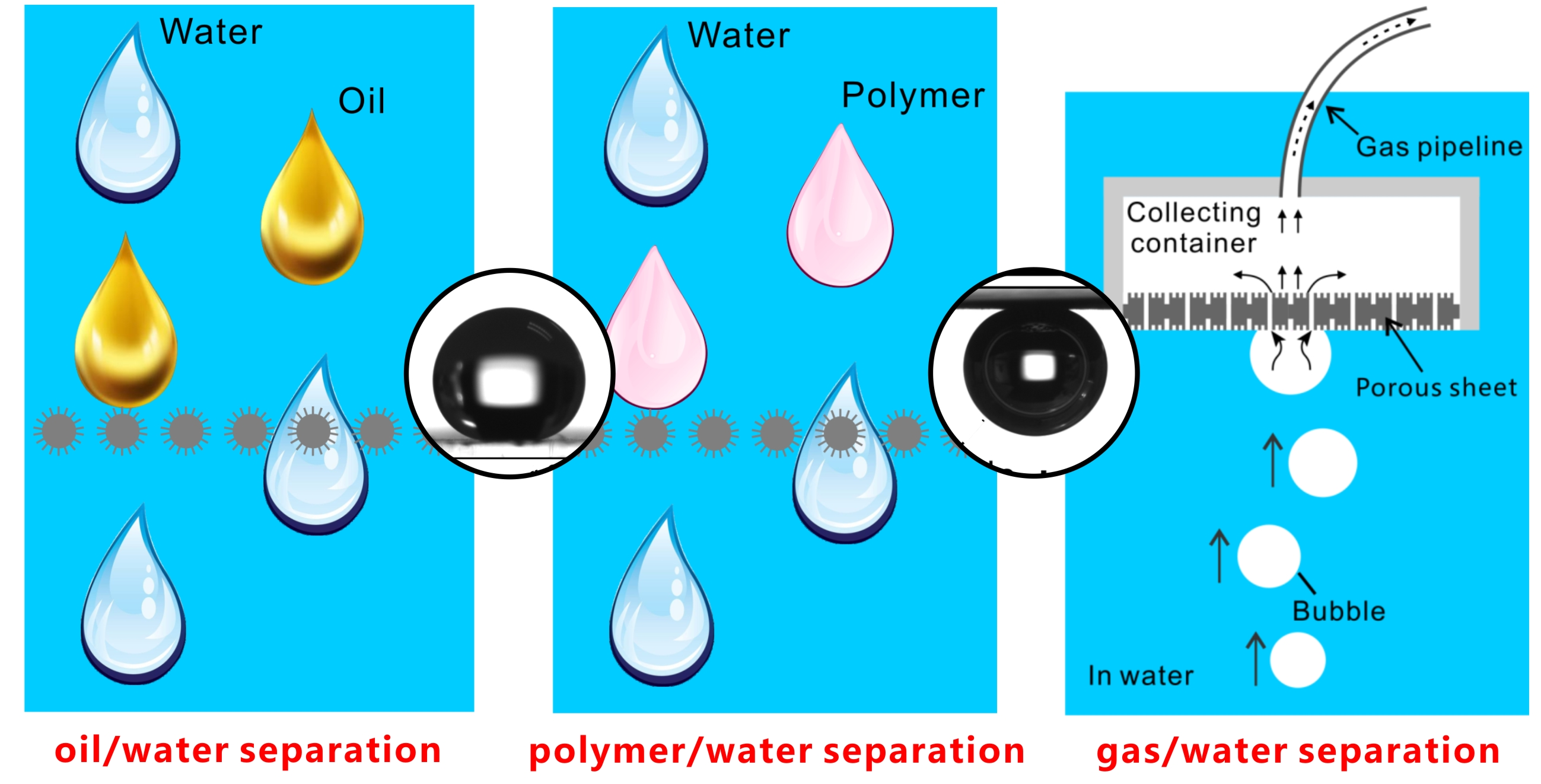
Nanomaterials, Free Full-Text