2) 1:12:15 (3) 12:15: Jals (4) 2 5 The compressibility factor nitrogen 330 K and 800 atm is 1.90 and 200 atm is 1.10.A certain mass of Noccupies a volume of 1

Click here:point_up_2:to get an answer to your question :writing_hand:2 112153 1215 jals 42 5the compressibility factor for nitrogen at 330 k and 800
Click here👆to get an answer to your question ✍️ -2- 1-12-15 -3- 12-15- Jals -4- 2 5 The compressibility factor nitrogen 330 K and 800 atm is 1-90 and 200 atm is 1-10-A certain mass of Noccupies a volume of 1 dmat 330 Kand eoo atm calculate volume occupied by same cuany of gas 750 K and 200 atm- -1- 1 L -2- 2L -3- 3L

The compressibility factor for nitrogen at 330K and 800 atm is 1.90 an
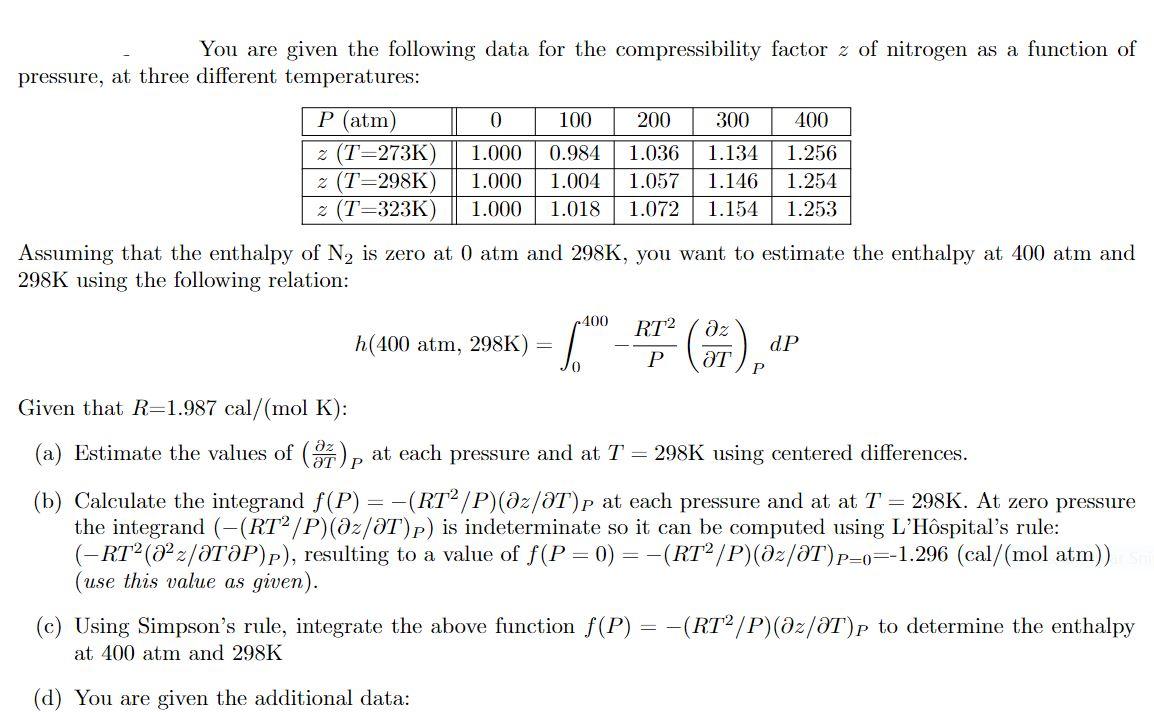
Solved You are given the following data for the

Solved 3.36) Determine the compressibility factor for

The compressibility factor for nitrogen at 330 K and 800 atm is

Compressibility factor Z for N 2 at 23∘ C and 821 atm is 1.25
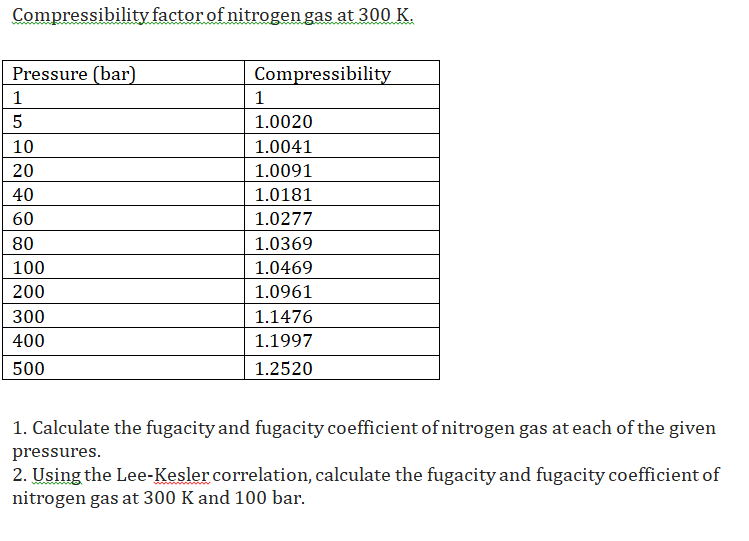
Solved Compressibility factor of nitrogen gas at 300 K.
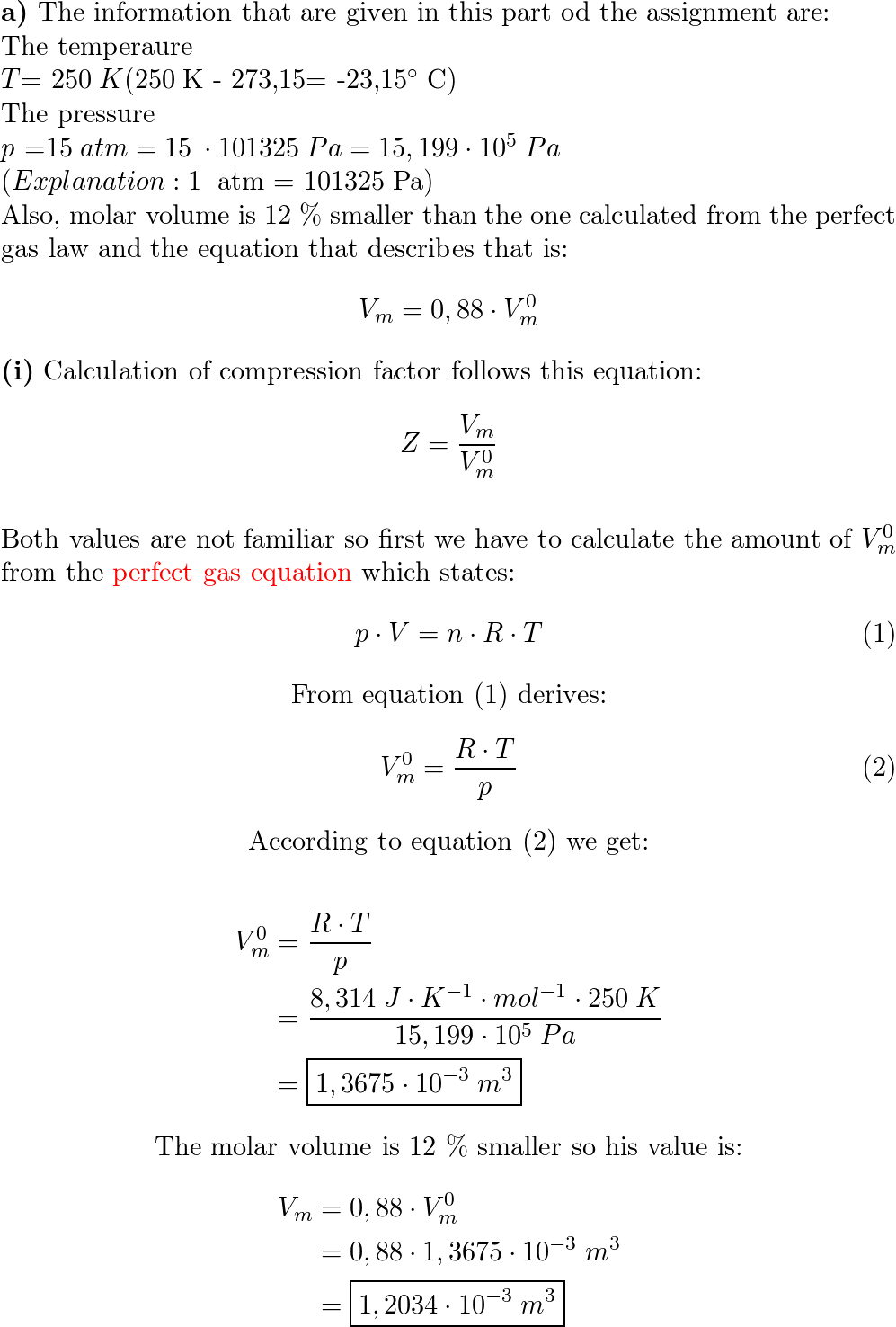
a) A gas at 250 K and 15 atm has a molar volume 12 per cent

The comperessibility factor for N(2) at -50^(@) C and 800 atm pressur

At total pressure P_1 atm and P_2 atm N_2O_4 is dissociated to an
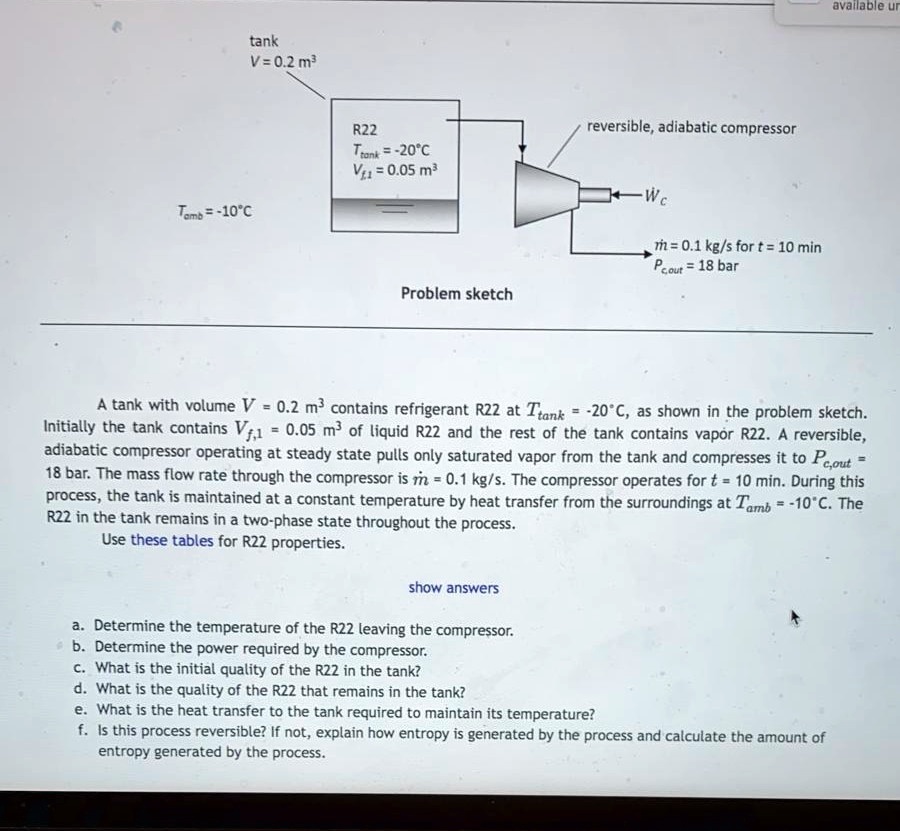
SOLVED: Text: available u tank V=0.2 m^3 R22 Tan=-20°C V=0.05 m^3

Advanced Thermodynamics Note 1 The 1st law and other basic
The compressibility factor for nitrogen at 330K and 800 atm is 1.90 an
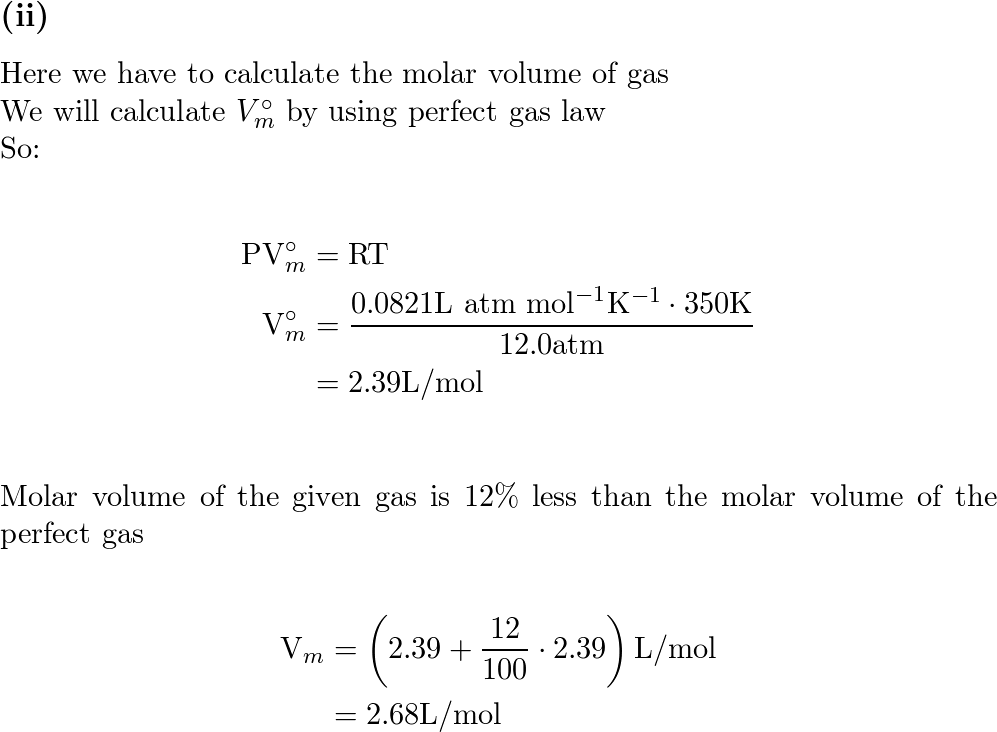
a) A gas at 250 K and 15 atm has a molar volume 12 per cent
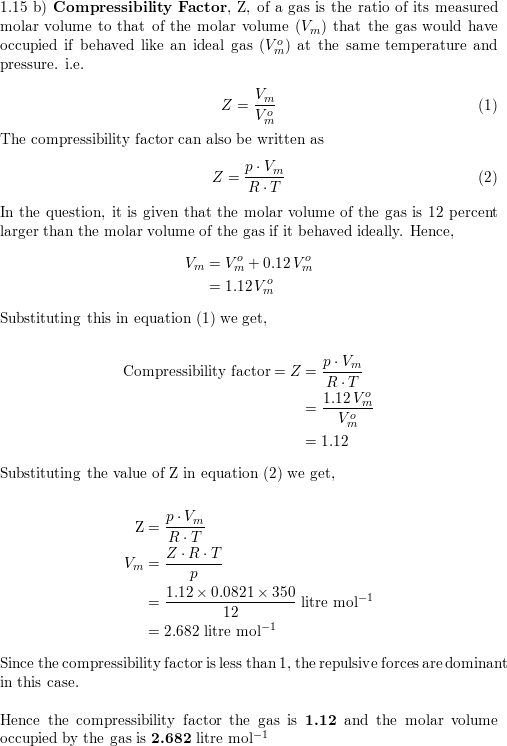
a) A gas at 250 K and 15 atm has a molar volume 12 per cent

Solved 2) Two liters of N2 at 0°C and 5 atm pressure are