Solved 3.30 The density of water is 1.00 g/mL at 48C. How


Calculating Density Warm Up What is the density (g/cm3) of 48.0 g

Chem.116 qualitative chemistry problem set

Number of Molecules in One Liter of Water
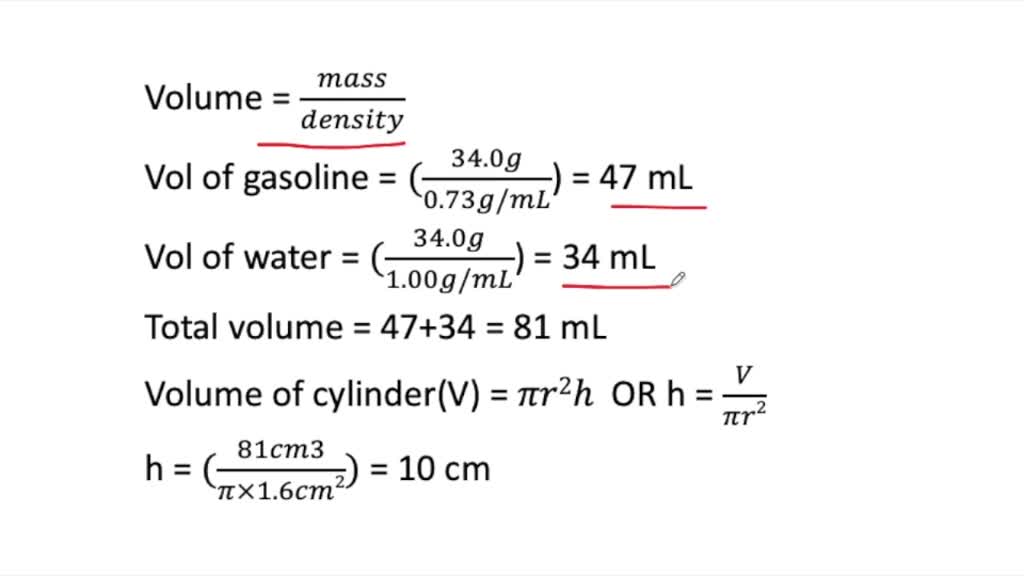
⏩SOLVED:Gasoline and water are immiscible. Regular-grade (87

Unit 4: Solution Calorimetry
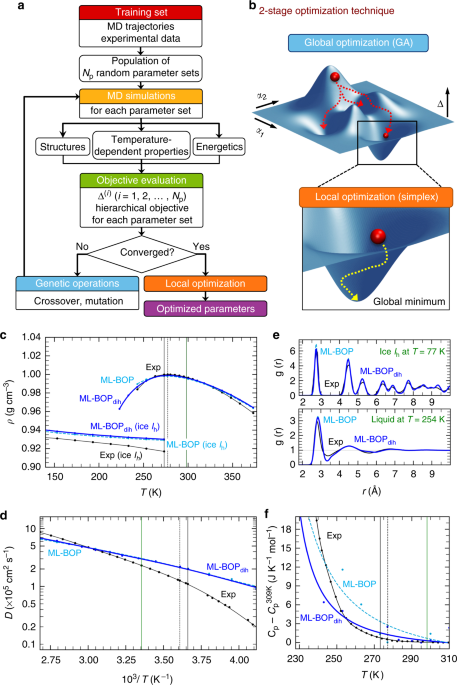
Machine learning coarse grained models for water

Solved 2. Assuming that the density of water is 1.00 g/ml

Density of water is 1 g/ mL . The concentration of water in mol
How are you able to calculate molarity from density? - Quora
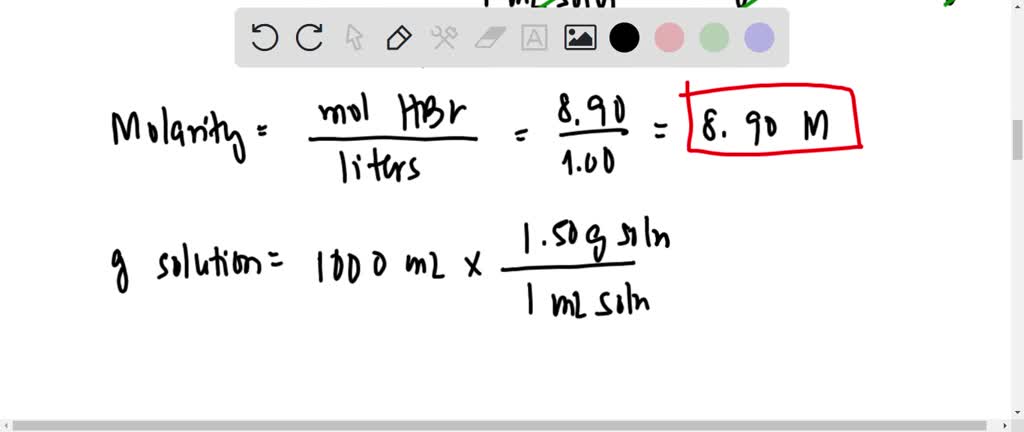
SOLVED: solution of HBr in water has density of 1.50 g/mL 48.0 wt
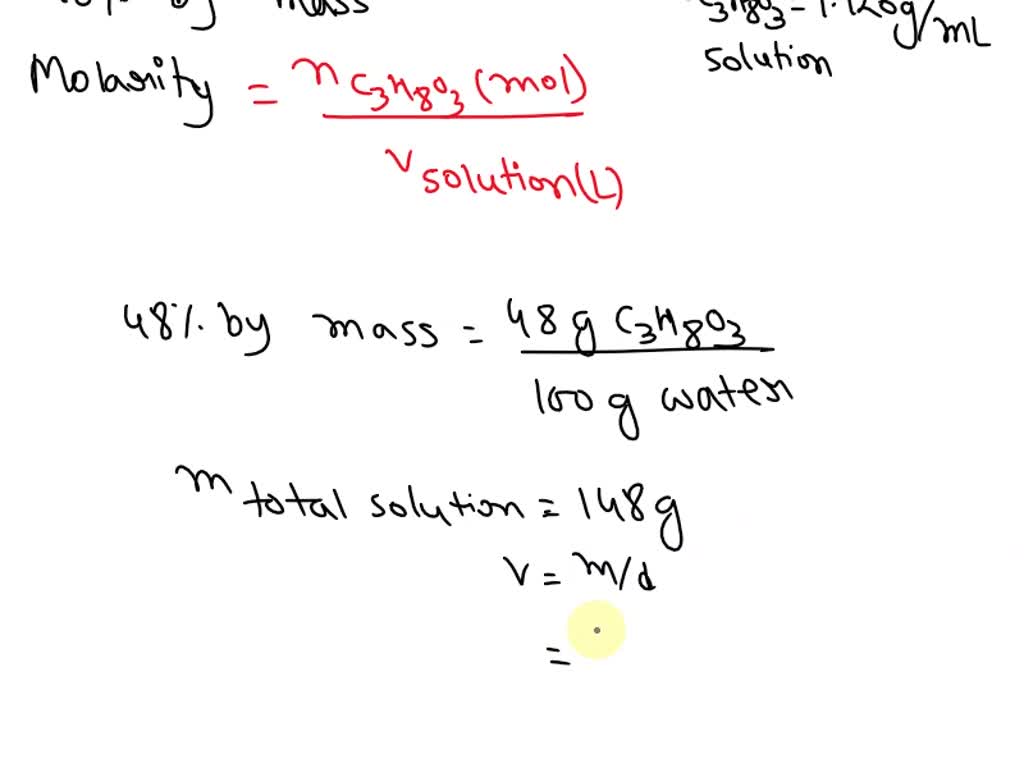
SOLVED: An aqueous solution of glycerol, C3H8O3, is 48.00

Solved The density of water at 30.0 °C is 0.9956 g/mL. If

Molarity Calculations