42g of N₂ react with excess of O₂ to produce NO. Amount of NO formed is a.60g b.32g c.45g d.90g

Share your videos with friends, family and the world
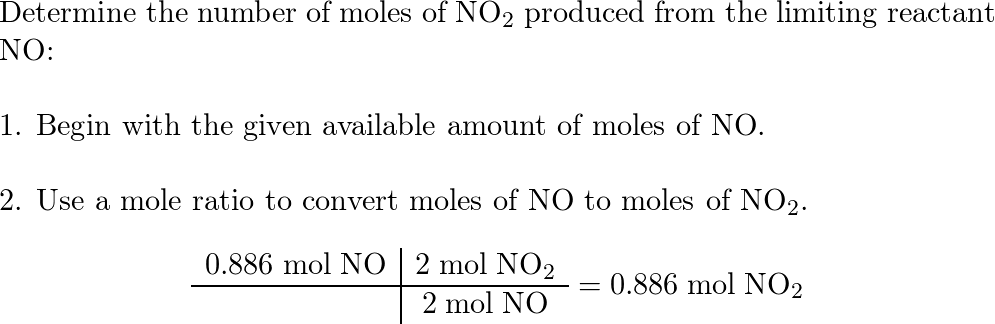
Nitric oxide (NO) reacts with oxygen gas to form nitrogen di

Topical Mock Chemistry Questions, PDF

Consider the reaction between NO(g) and O2(g) represented below
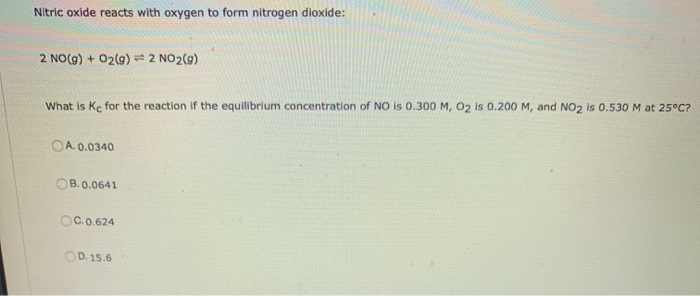
Solved Nitric oxide reacts with oxygen to form nitrogen
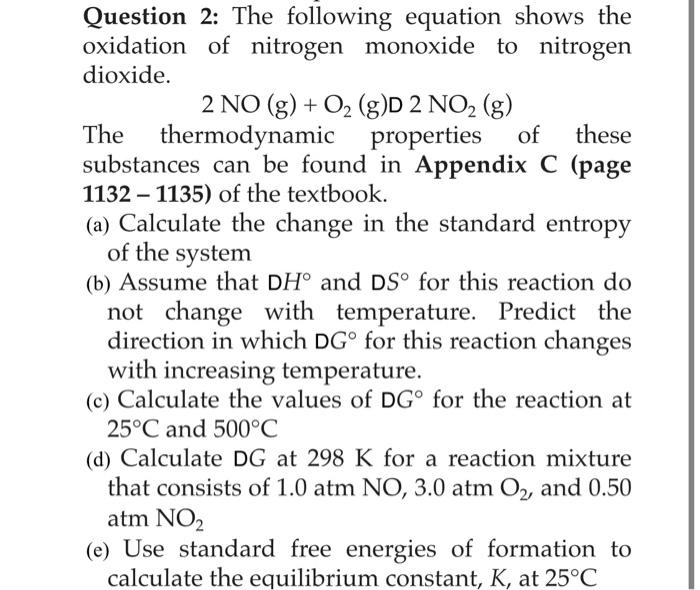
Solved Question 2: The following equation shows the

Solved For the following reaction, 34.6 grams of sulfuric

Solved Identify the limiting reactant in the reaction of

Solved Nitrogen and oxygen react as follows: N2(g) +202(g) +
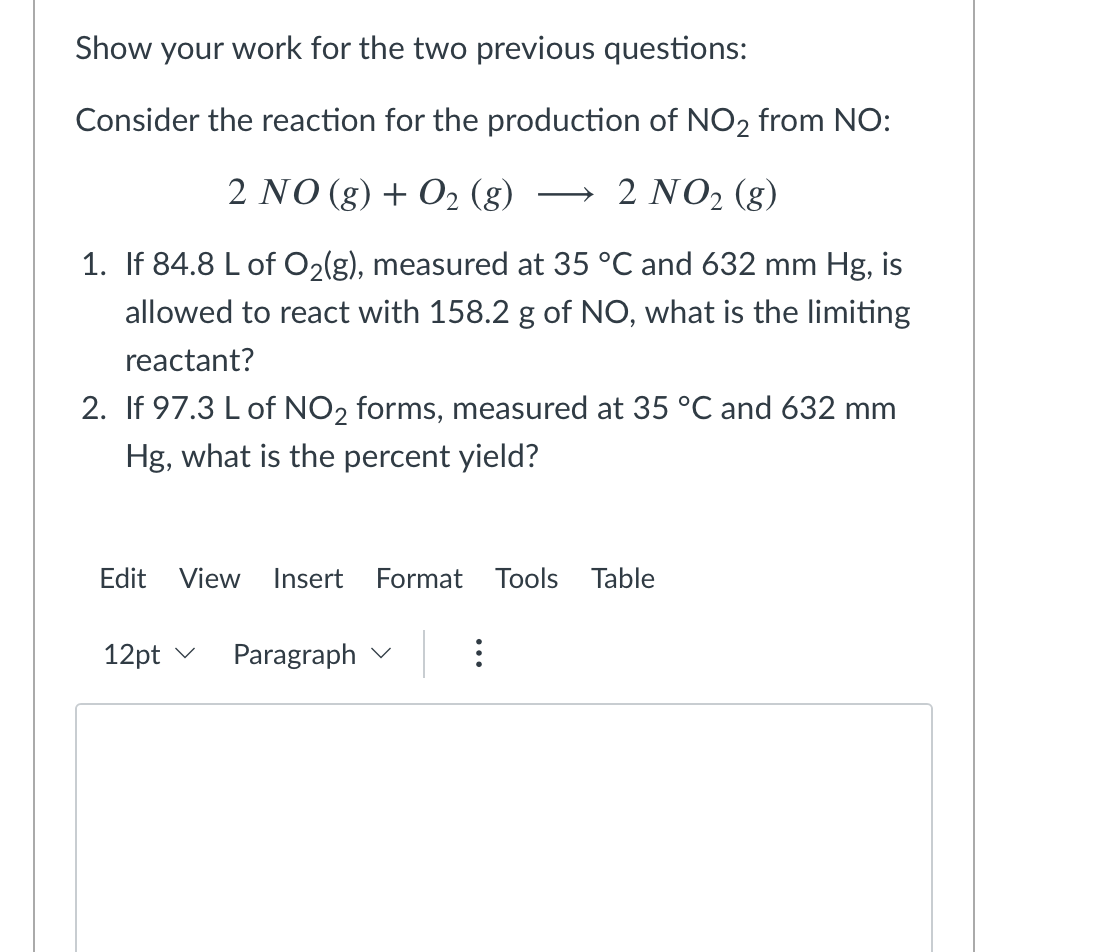
Solved Show your work for the two previous questions

JP2022531876A - Convergent liquid phase synthesis of

Topical Mock Chemistry Questions, PDF

Solved For the following reaction, 10.2 grams of carbon

Nitric oxide (NO) reacts with oxygen gas to form nitrogen di

Solved For the following reaction, 19.9 grams of nitrogen
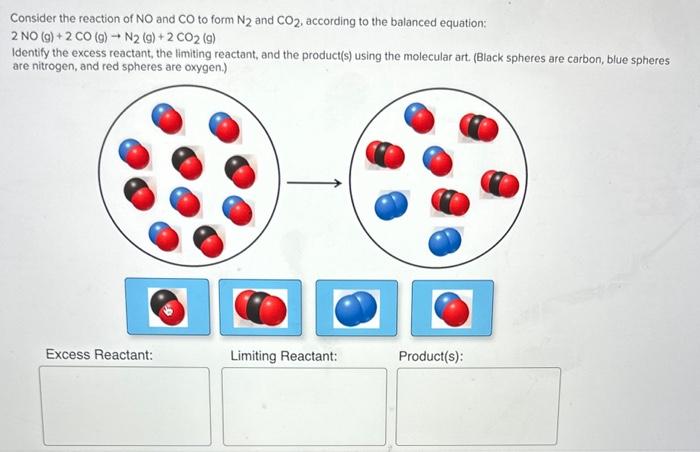
Solved Consider the reaction of NO and CO to form N2 and