FDA Approves Senza®, Nevro's High Frequency Spinal Cord
The Senza System has been approved by the FDA for the treatment of chronic pain associated with painful diabetic neuropathy.

Elon Musk Reveals he Used Novo's Wegovy for Weight Loss + FDA Approval of Nevro's AI Spinal Cord Stimulation System – Xtalks Life Science Podcast Ep. 82 - Xtalks
HF10 Therapy Shown Effective For Variety of Pain Conditions
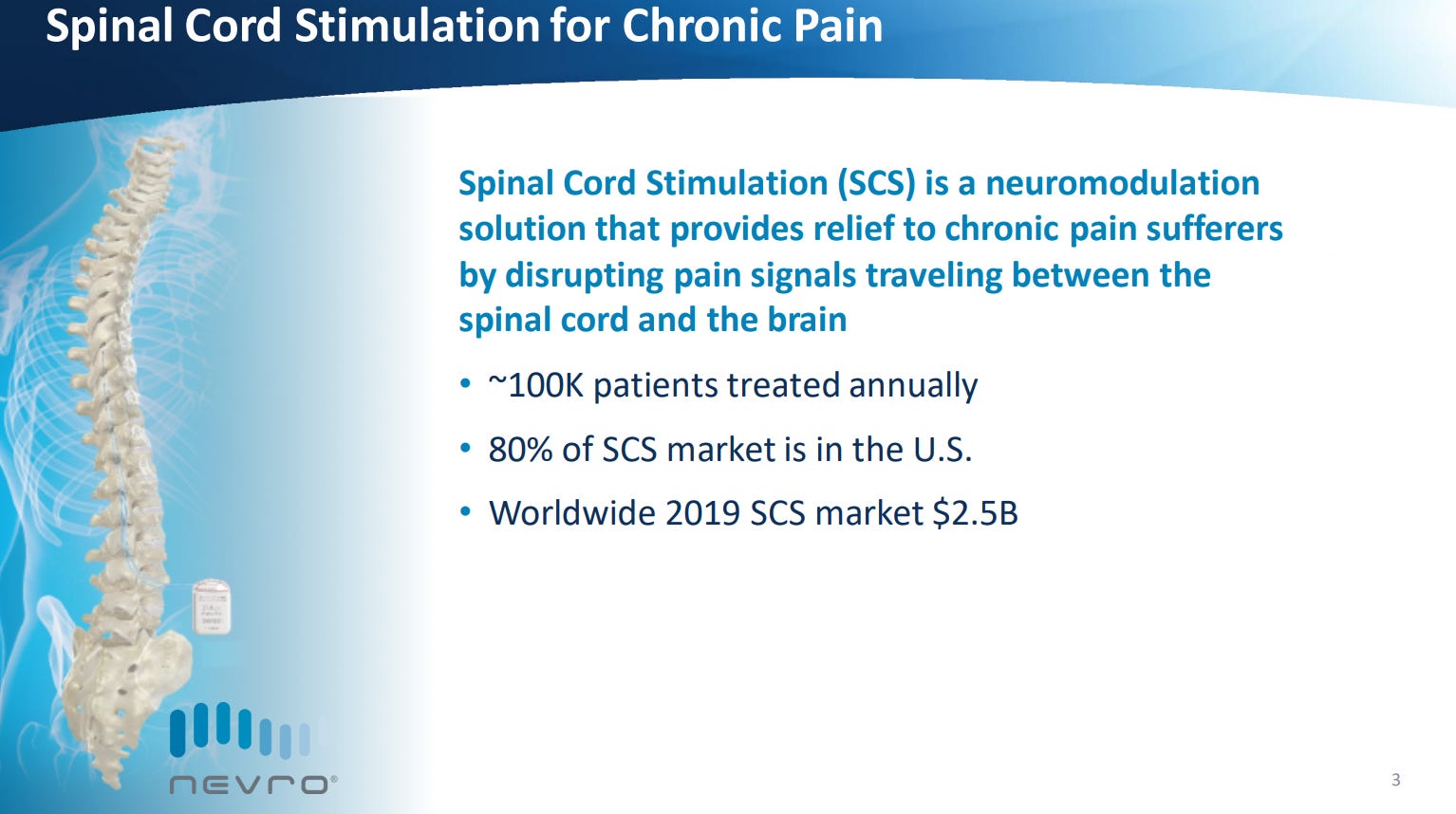
Deck Review with Nevro - by Joshua Elkington - Axial

Nevro's Spinal Cord Stimulation System Efficacious in Treating Painful Diabetic Neuropathy

Position Statement on Spinal Cord Stimulation for Patients with Painful Diabetic Neuropathy — OHSIPP

Nevro Receives FDA Approval For Senza II Spinal Cord Stimulation System Delivering HF10 Therapy
NANS 2021: Nevro's HF10 Spinal-Cord Stimulator Succeeds In Two Trials :: Medtech Insight
PDF) High-Frequency 10·kHz Spinal Cord Stimulation for Chronic Back and Leg Pain: Cost-consequence and Cost-Effectiveness Analyses
Nevro receives CE mark for full-body MRI conditional labelling with the Senza system - NeuroNews International
Spinal Stimulation Therapy, Chronic Pain Relief

Wireless Spinal Cord Stimulator Cleared by FDA

FDA accepted Nevro's Senza II for chronic pain treatment – Meba Mercati Balcanici
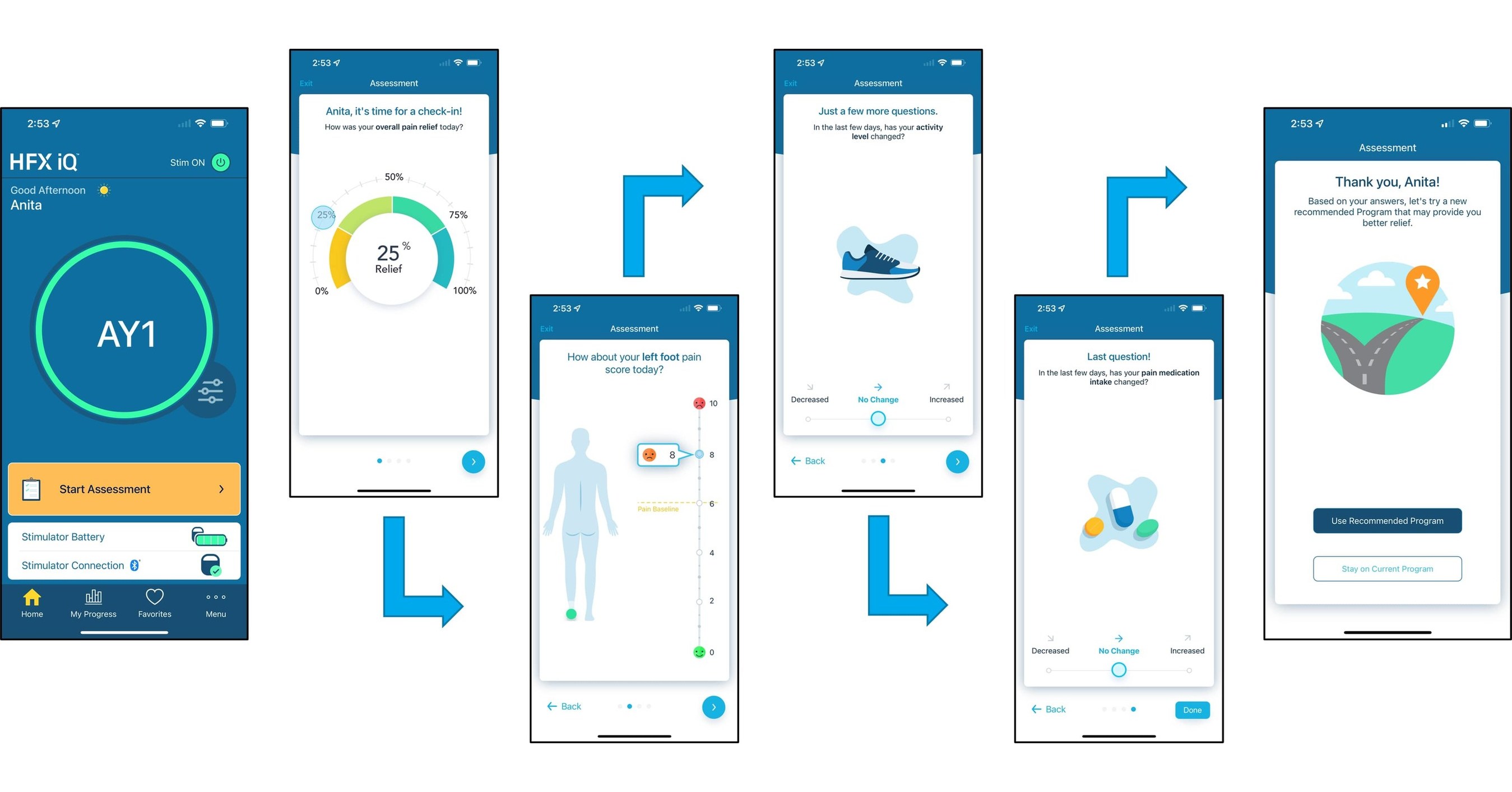
Nevro Announces FDA Approval of HFX iQ™ Spinal Cord Stimulation System to Personalize the Treatment of Chronic Pain

Nevro Corp. - Nevro Announces FDA Approval of its 10 kHz High Frequency Spinal Cord Stimulation Therapy for Treatment of Chronic Pain Associated with Painful Diabetic Neuropathy (PDN)

10-kHz spinal cord stimulation treatment for painful diabetic neuropathy: results from post-hoc analysis of the SENZA-PPN study