At a high pressure, the compressibility factor (Z) of a real gas is us

At high P. P gt gt (n^(2)a)/(V^(2)) So ‘a’ can be neglected.
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas

Gas Compressibility Factor and Control Valve Sizing

Real gas z-Factor chart [2] Download Scientific Diagram
Ideal gases and real gases are compressible or not compressible what is the compressible factor for real gases and ideal gases.
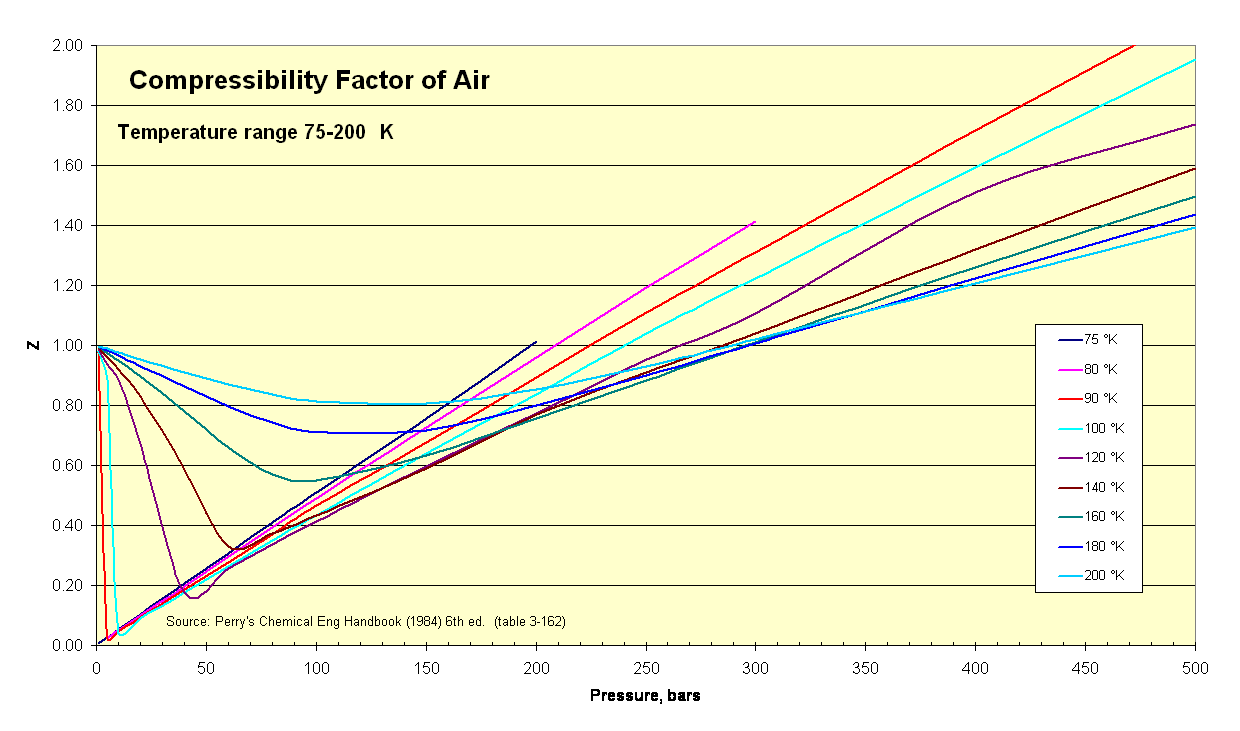
Air Compressibility Factor Table - EnggCyclopedia

Value of Compressibility Factor (z)at low pressure and high pressure(JEE Mains 2014) Q. & A

At a high pressure, the compressibility factor (Z) of a real gas is us

Real Gas Behavior The Compression Factor (Z) [Example #2]

Real gas z-factor, as attributed to Standing and Katz, 9 plotted as a
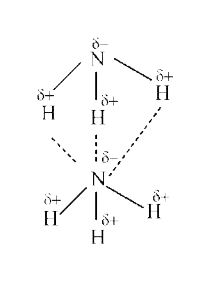
Which pair of molecules has the strongest dipole – dipole interactions

e Compressibility factor (Z) for hydrogen WRT pressure and temperature
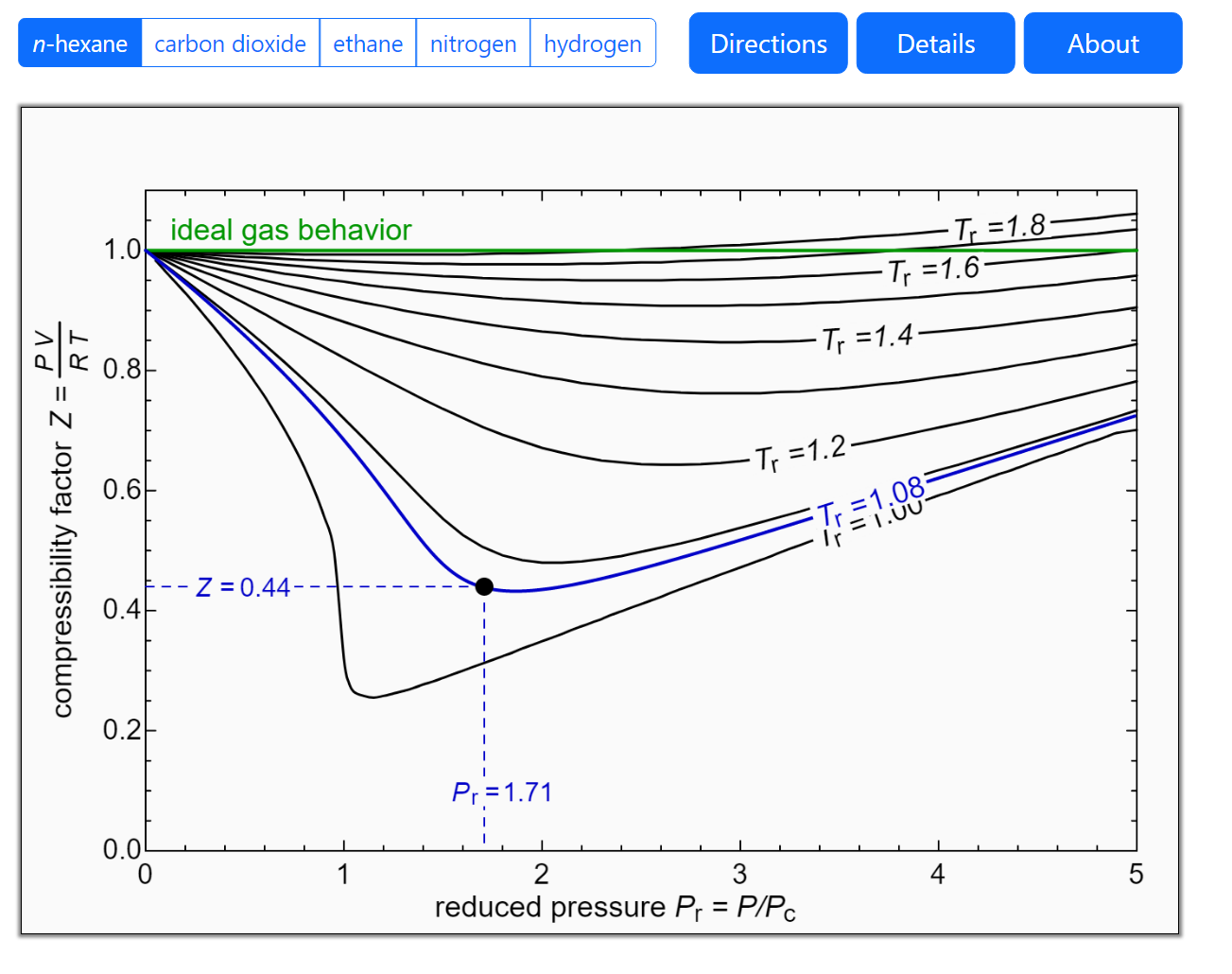
/wp-content/uploads/2023/05/compress

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

Determine Compressibility of Gases

A : At high pressure , the compressibility factor Z is (1 + (pb)/(RT))