Draft Guidance Document: Applications for Medical Device Investigational Testing Authorizations

This draft guidance document reflects Health Canada’s current thinking on Investigational Testing Authorizations (ITA) for medical devices and may be subject to changes as policy develops. The document clarifies application requirements and processes, including pre-ITA meetings, format for an ITA application and filing requests for revisions to an ITA.
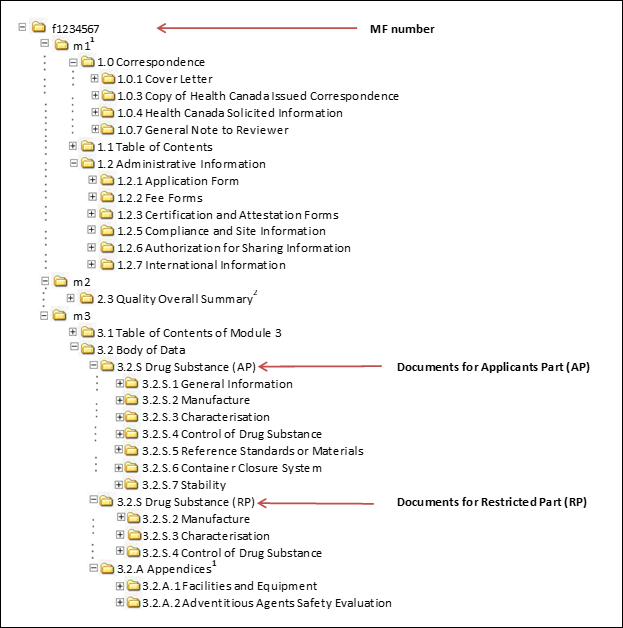
Guidance document: preparation of regulatory activities in non-eCTD format
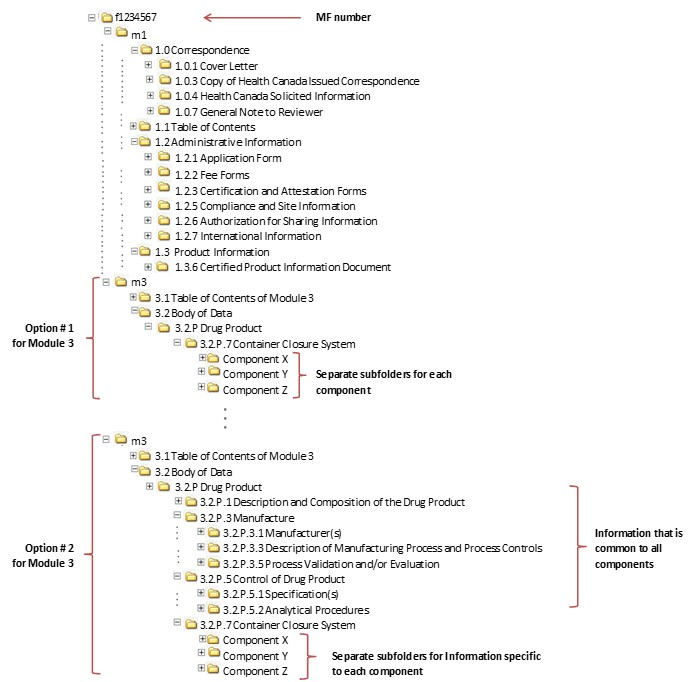
Guidance document: preparation of regulatory activities in non-eCTD format

Draft guidance for determining medical device application type: Overview

Canada's Health Canada - Global Regulatory Partners, Inc.

Applications for Medical Device Investigational Testing Authorizations Guidance Document

ESMO Guidance for Reporting Oncology real-World evidence (GROW) - ESMO Real World Data and Digital Oncology
The FDA Regulatory Landscape For AI In Medical Devices
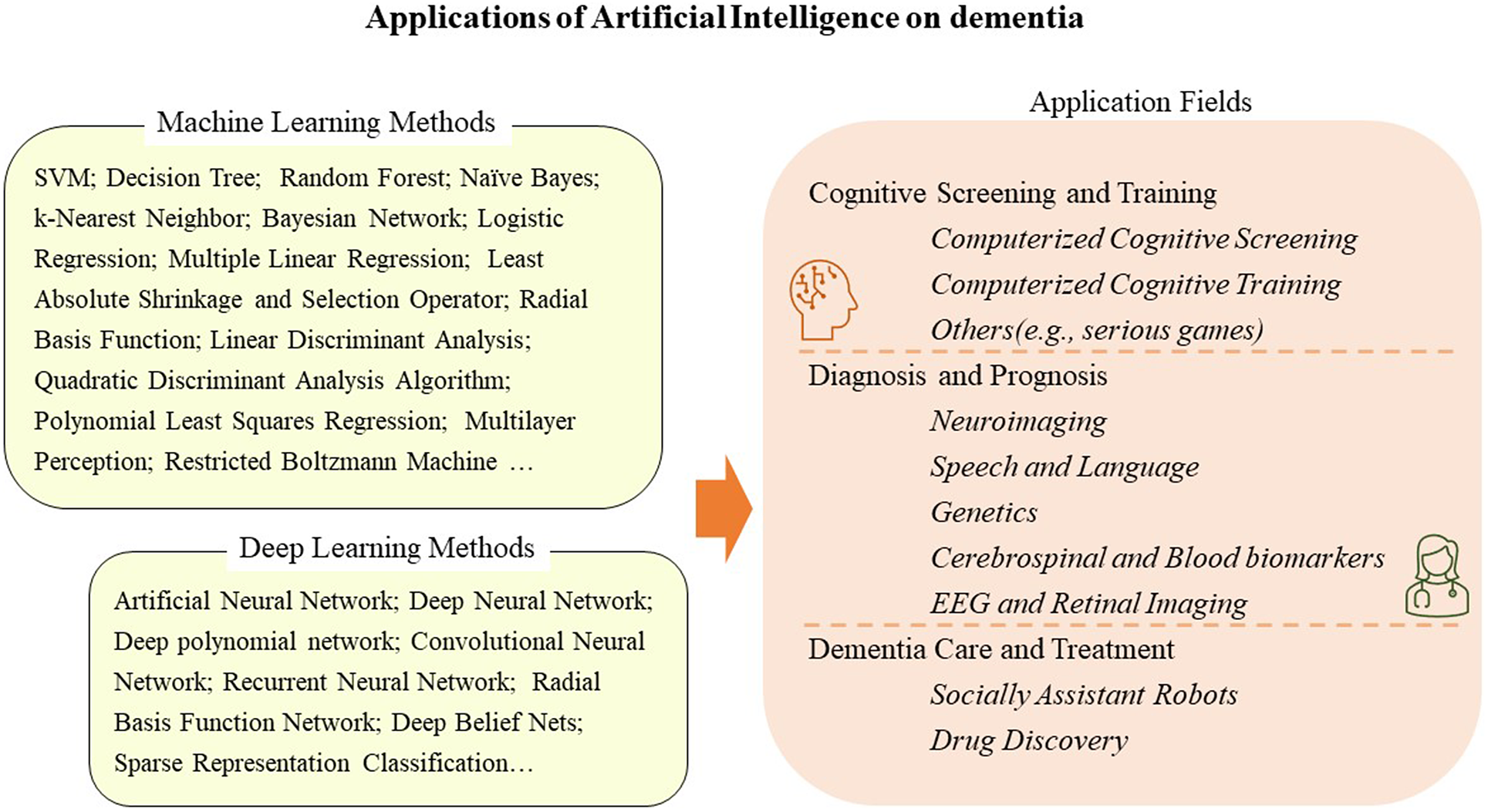
Applications of artificial intelligence in dementia research, Cambridge Prisms: Precision Medicine
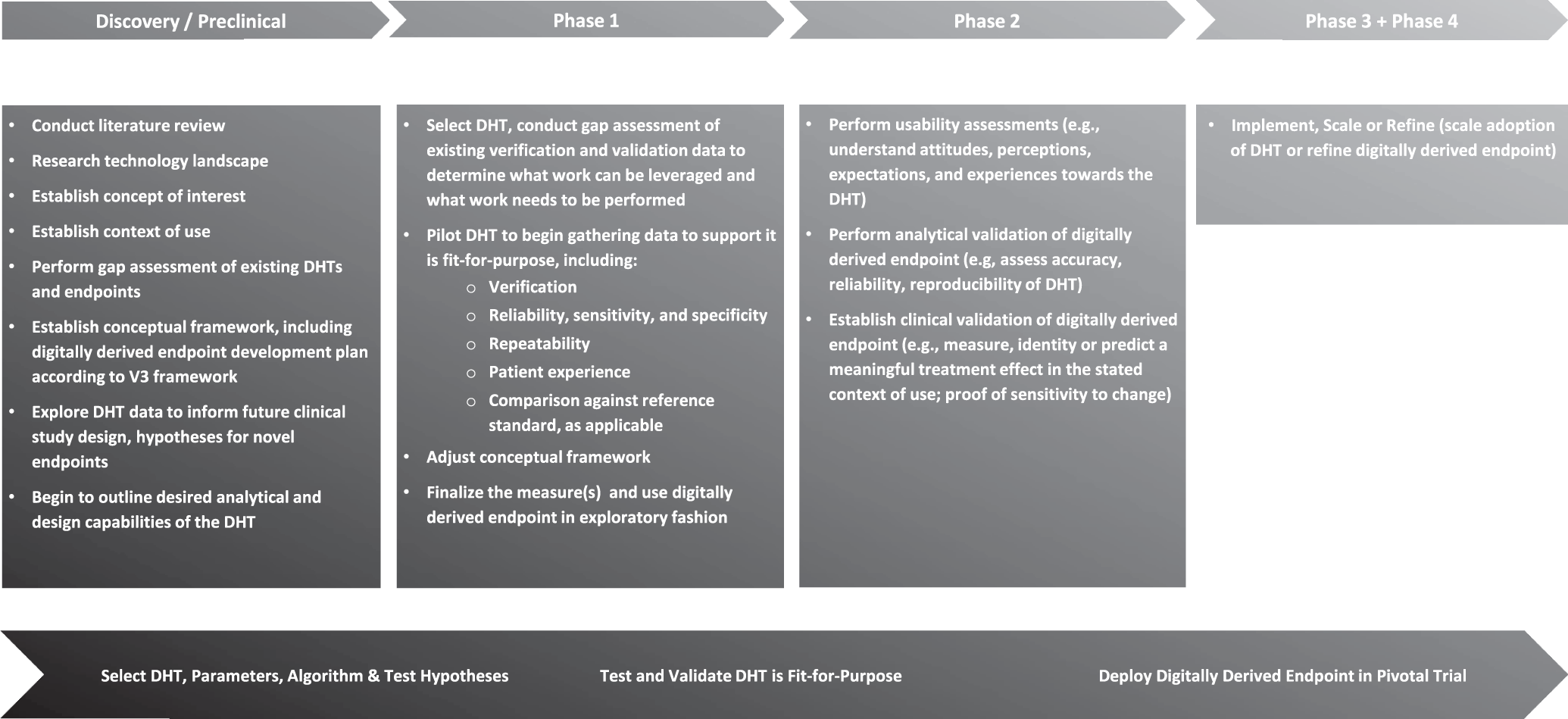
Incorporating digitally derived endpoints within clinical development programs by leveraging prior work
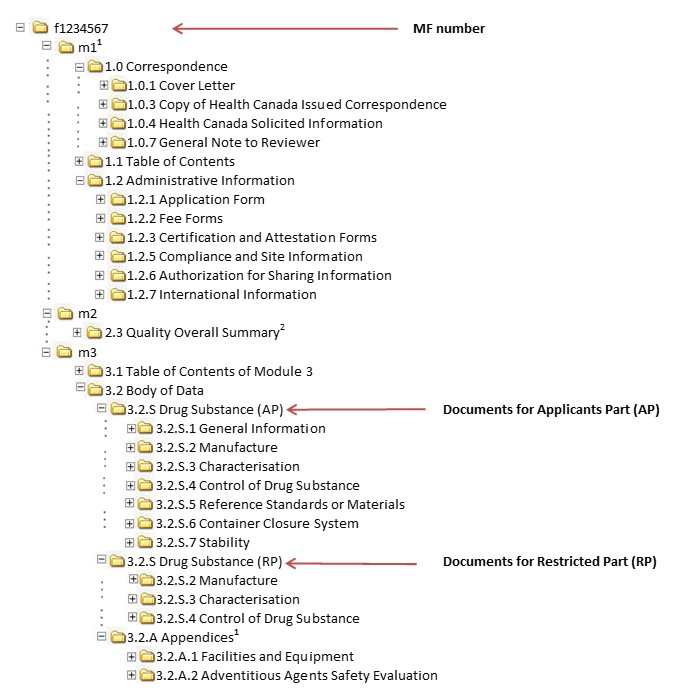
Guidance document: preparation of regulatory activities in non-eCTD format

Product-Specific Guidances for Generic Drug Development

FDA 2022 annual report shows steady rate of medical device submission reviews
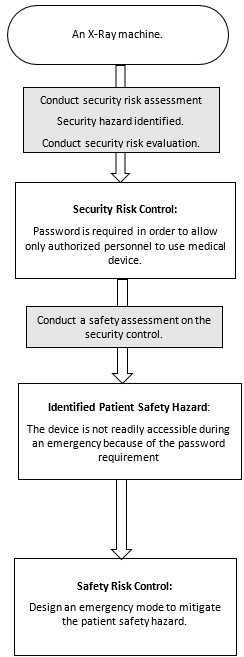
Guidance Document: Pre-market Requirements for Medical Device Cybersecurity

De-identification of Protected Health Information: 2024 Update

Steps involved in US FDA's Medical devices Validation Protocol Process