Establishing expiry date for clinical diagnostic reagents

Product shelf life is an essential product performance requirement that, along with other design requirements, is used to determine the safety and efficacy of a clinical diagnostic

How to Handle Lab Reagents After Their Expiration Date

Mark Miller on LinkedIn: #diagnosticsispower

THENA Capital on LinkedIn: #grit #determination #femalefounders
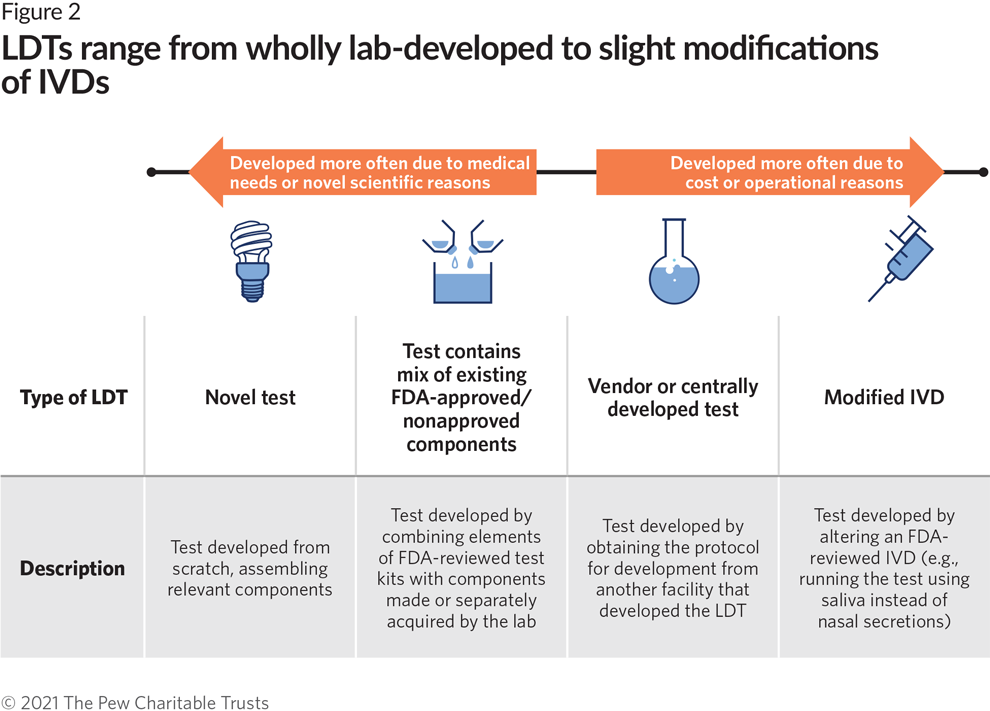
Diagnostic Tests Not Reviewed by FDA Present Growing Risks to
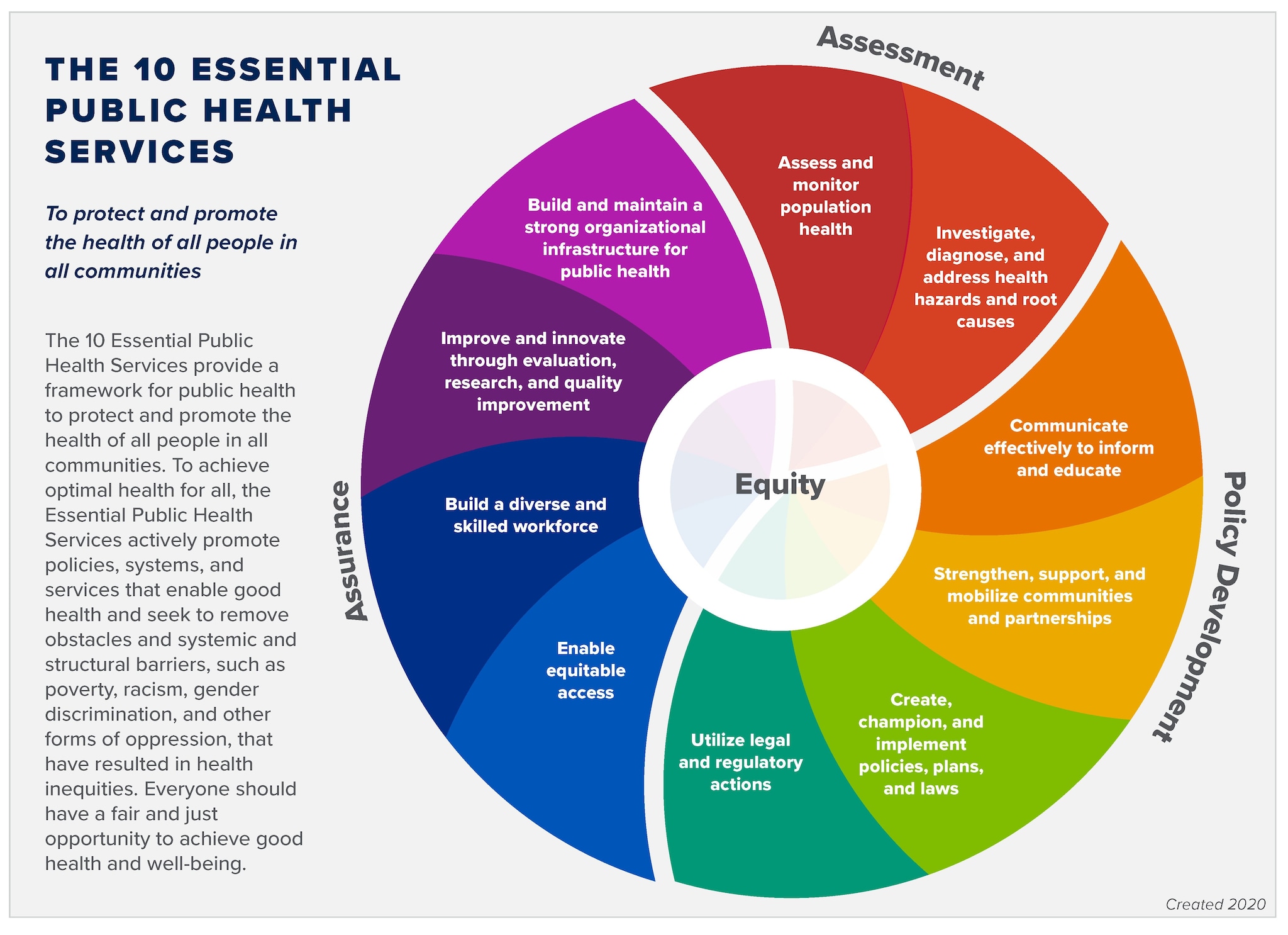
CDC - 10 Essential Public Health Services - Public Health
Industry Reacts to FDA Draft Guidance on Injectable Products

Diagnostic Reagent, Medical Reagents Manufacturer/Supplier

Laboratory Handling Controls

Phases in the development of plant clinics and the Plant Health

How to estimate the expiration date of reagents made in the

At-Home OTC COVID-19 Diagnostic Tests