Ideal Gas Assumptions - Kinetic Theory

When considering a gas as an ideal gas and applying the ideal gas law pV=nRT, we need to make 4 assumptions. (1) The volume of a molecule within the gas is n
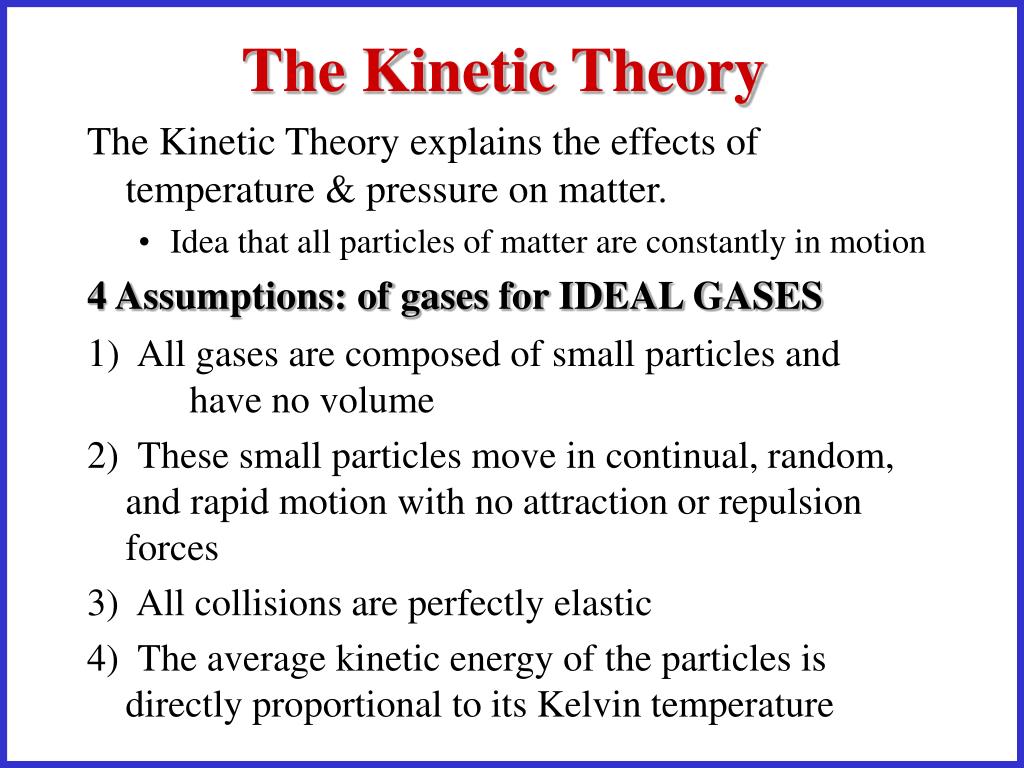
PPT - The Kinetic Theory, Pressure & Gas Laws PowerPoint

Thermodynamics. The Ideal Gas Law Assumptions The particles of a

P7 - Gas Laws (Molecular Model and Kinetic Theory)

Physics 32 Kinetic Theory of a Gas (10 of 10) Time Between Collision
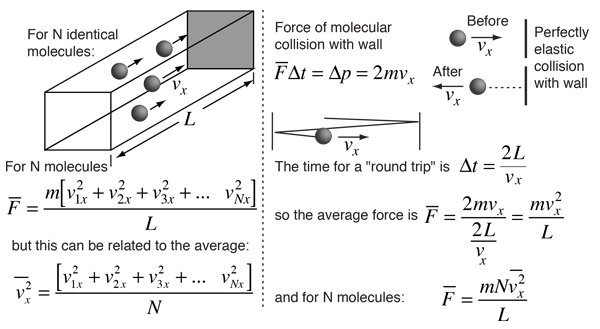
Kinetic Theory

The Kinetic-Molecular Theory of Gases

Cameroon General Certificate of Education Compress, PDF, Heat

Boyles' and Charles' Laws

IIT JEE and NEET Physics: Kinetic theory of gases and Expression

Solved 11 Which of the following assumptions is NOT * ?made

1 Ch 10.1 Kinetic Theory: 5 assumptions 1.small particles - far

Kinetic theory of gases - Wikipedia

Physics 32 Kinetic Theory of a Gas (10 of 10) Time Between Collision