Ideal–Universal Gas Law
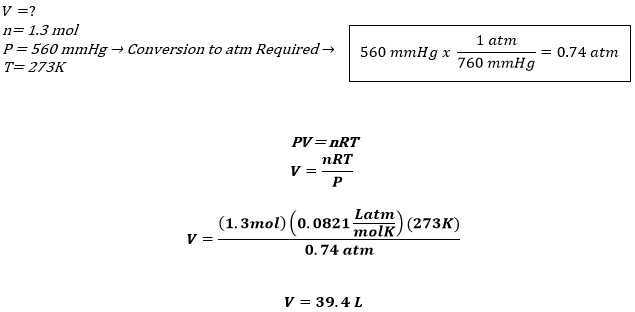
Definition: The Universal or Ideal Gas Law describes the relationship between all four properties (pressure, volume, number of moles, and temperature) as well as a gas constant called “R.” NOTE: The Ideal Gas Constant “R” has constant a value of 0.0821 L.atm/mol.K Relation: The relation between pressure (P) volume (V), number of moles (n) and…
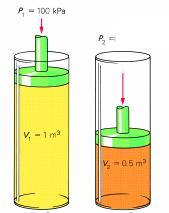
The Combined Gas Law

Electrolytes
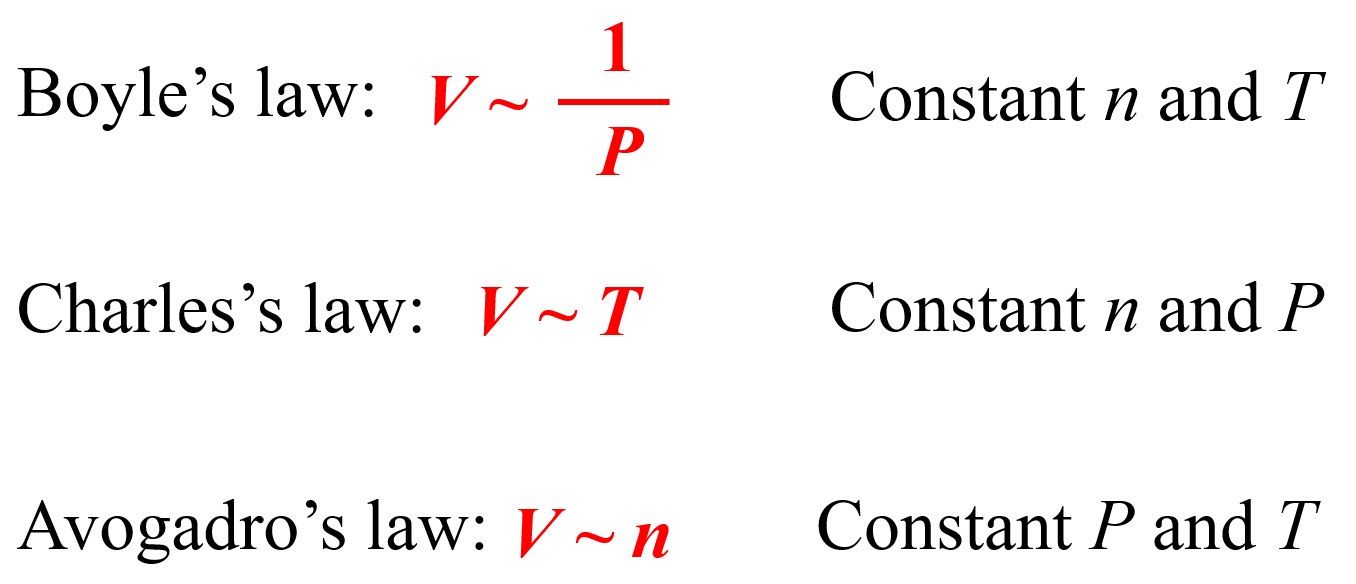
The Ideal Gas Law - Chemistry Steps
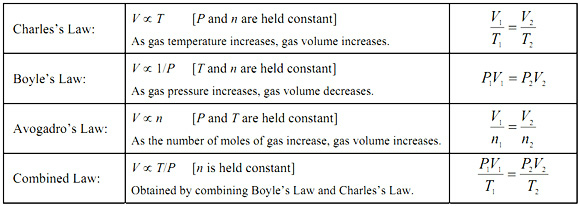
What does the ideal gas law allow a scientist to calculate that the other gas laws do not?

The Mole Concept: Molecules and Atoms
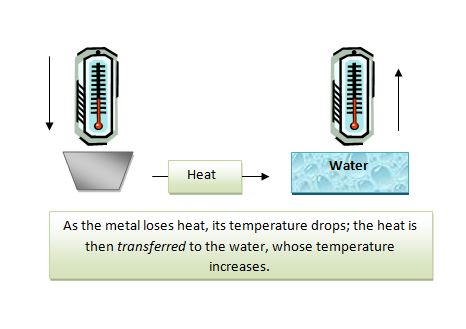
Calorimetry
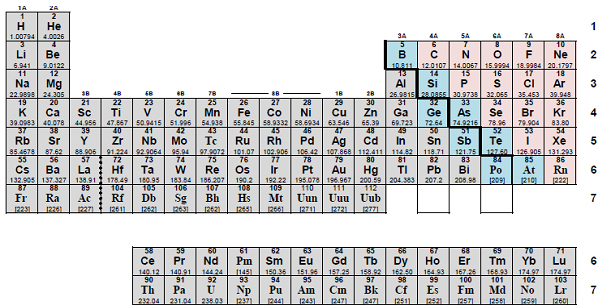
Anatomy of the Periodic Table

Gas Constant: Definition, Formula, Ideal Gas and Examples

Physics, Chemistry, Biochemistry Flashcards

The Activity Series