Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel

Groundbreaking technology introduces increased control to achieve optimal coverage proven to significantly reduce the risk of toxicity to the rectum SANTA BARBARA, CALIF. / STOCKHOLM, SWEDEN – June 9, 2022— Palette Life Sciences, a fully-integrated global life sciences company dedicated to improving patient outcomes, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of […]

CAROL THRONDSON on LinkedIn: Barrigel

Per Langoe on LinkedIn: This morning Teleflex Incorporated announced the acquisition of Palette…

Barrigel on LinkedIn: Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel® Rectal…

Dr. Martin King, MD – Boston, MA

SpaceOAR - Augmenix, Boston Scientific, and Conflicts of Interest, Page 4

Pratik Patel on LinkedIn: Great event
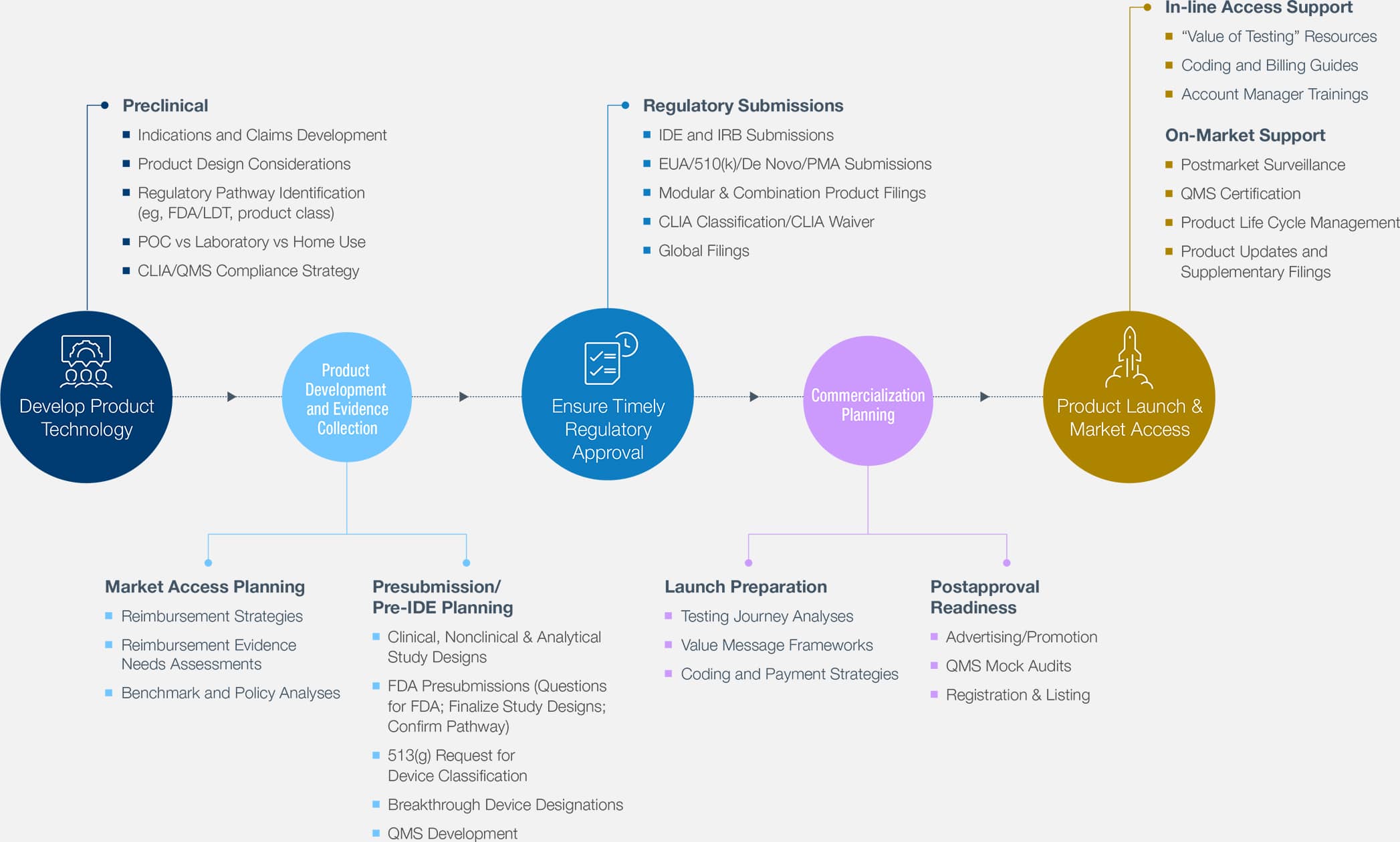
510(k) Covid-19 Assays Precision For Medicine

David Aguilar on LinkedIn: Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel® Rectal…
FDA Device Regulation: 510(k), PMA · Academic Entrepreneurship for Medical and Health Sciences

Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel® Rectal Spacer, Proven Safe and Effective at Minimizing the Harmful Long-Term Side Effects of Prostate Radiation Therapy - Palette Life Sciences

Helena Jansson på LinkedIn: Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel® Rectal…