Sacituzumab Earns Regular FDA Approval for TNBC - NCI

Sacituzumab govitecan (Trodelvy) now has regular FDA approval for people with locally advanced or metastatic triple-negative breast cancer (TNBC), including those with brain metastases. The update follows last year’s accelerated approval of the drug for people with TNBC.

FDA Grants Full Approval to Trodelvy for Triple-Negative Breast Cancer
Clinical Review - Sacituzumab Govitecan (Trodelvy) - NCBI Bookshelf

Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance

PDF) Analysis of patients without and with an initial triple

Mission Mountain Wilderness
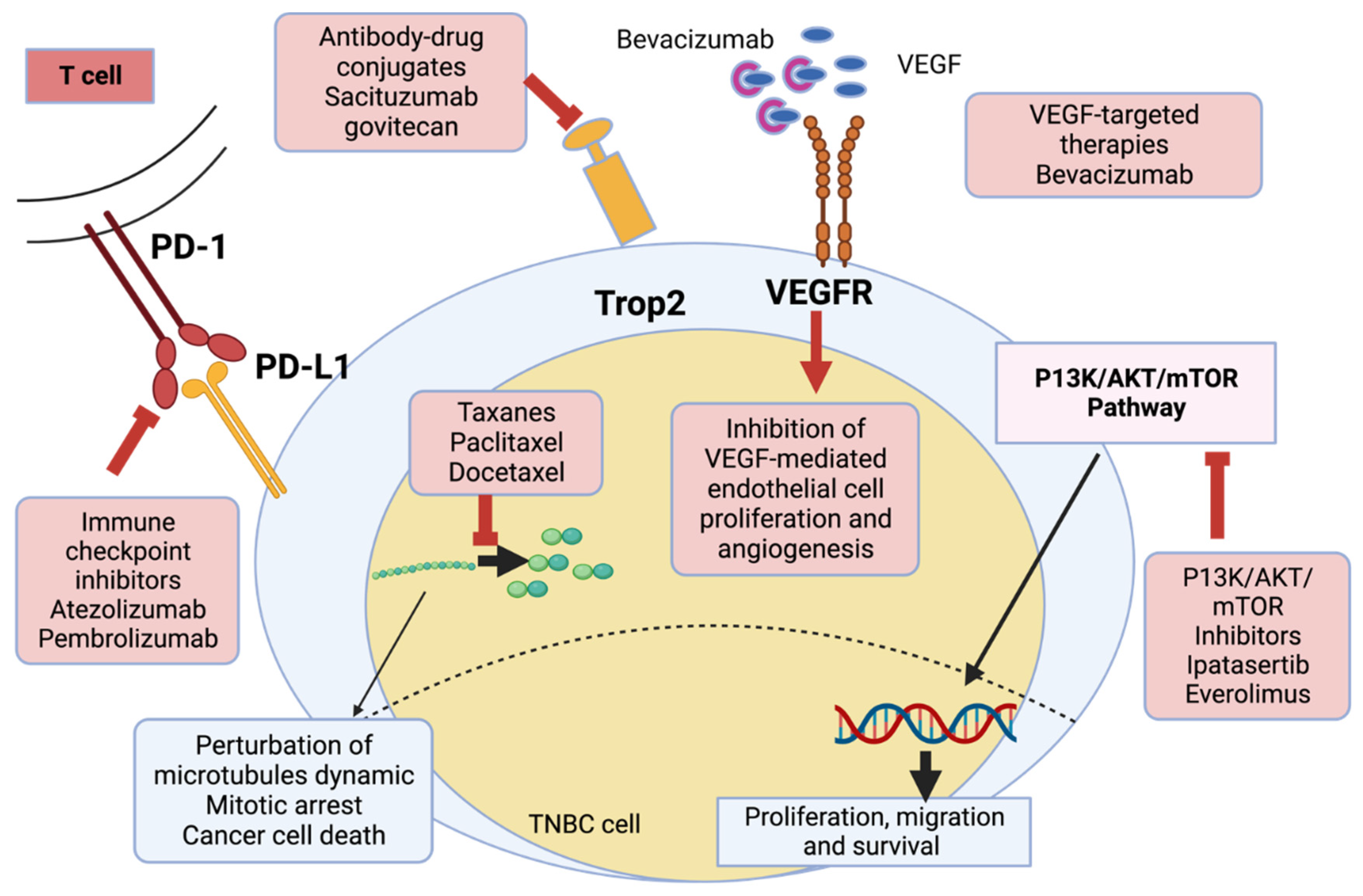
Pharmaceutics, Free Full-Text

ADC Drug Trodelvy Shows Positive Efficacy In three Types of Cancers
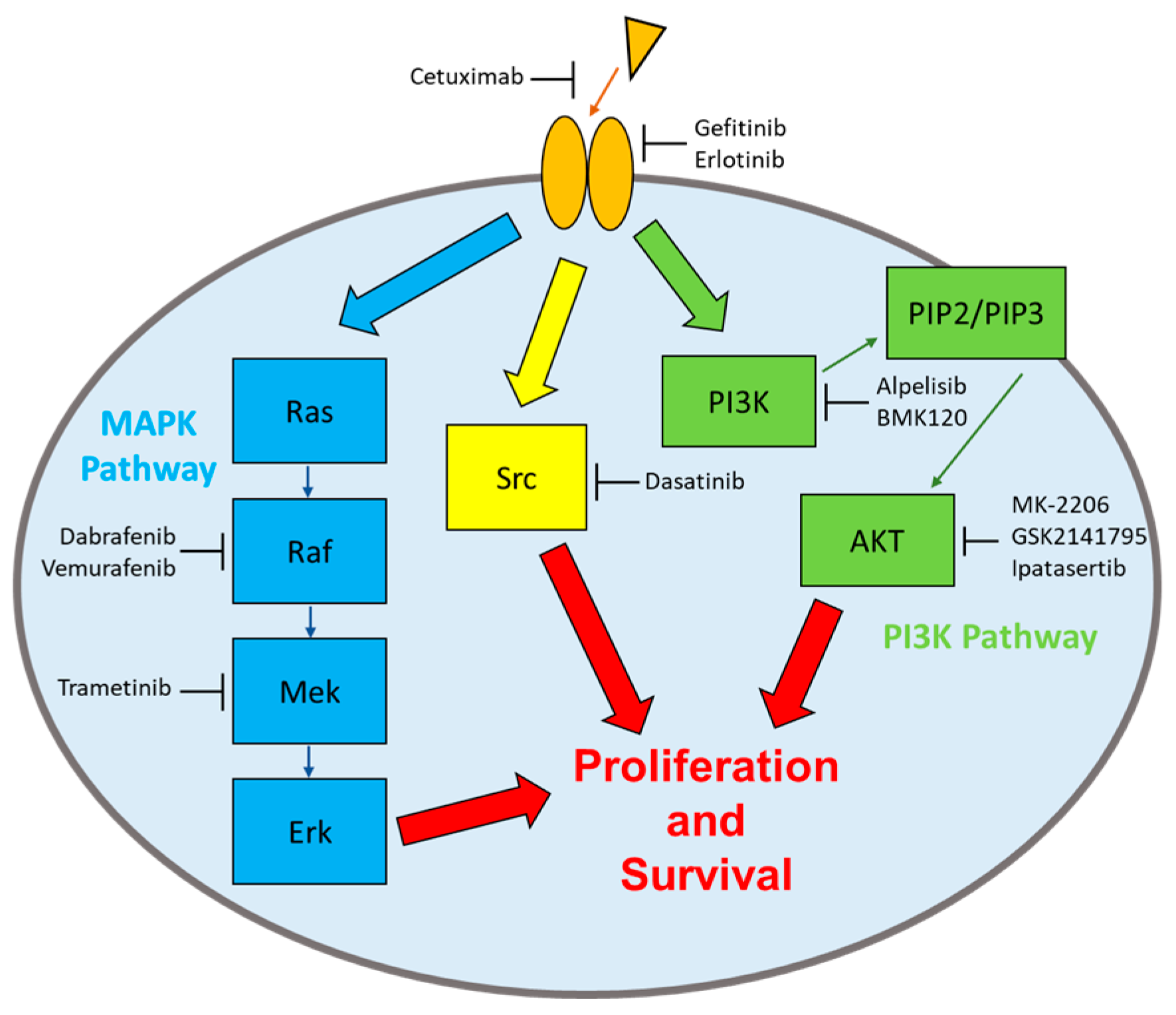
Biomolecules, Free Full-Text

PDF) Exploiting Therapeutic Vulnerabilities in Triple-Negative

Recap: The Use of Sacituzumab Govitecan in Triple-Negative Breast Cancer
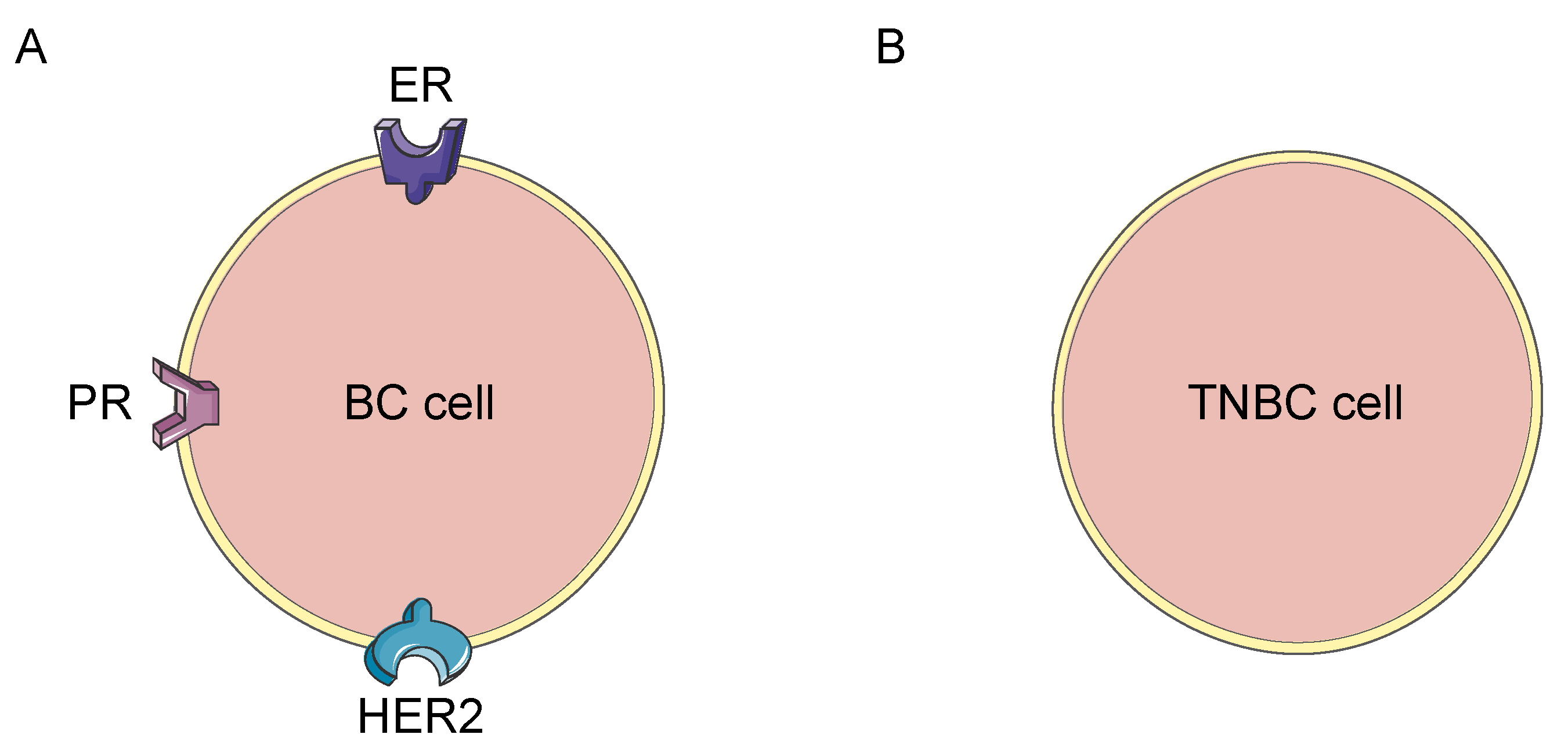
Sacituzumab Earns Regular FDA Approval For TNBC NCI