Solved 1. A cup of hot 50°C water is poured onto 35g of ice

Answer to Solved 1. A cup of hot 50°C water is poured onto 35g of ice
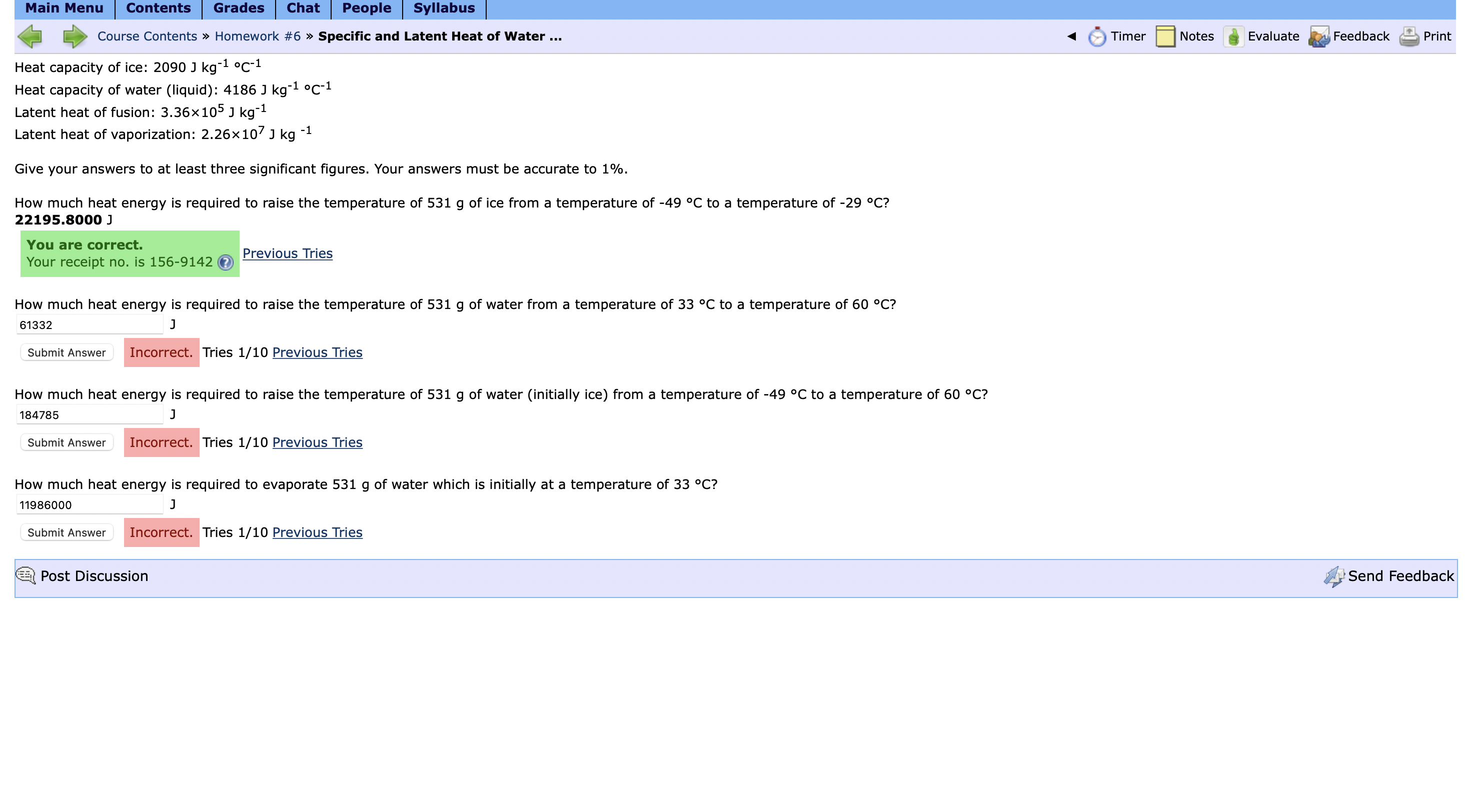
Solved Heat capacity of ice: 2090Jkg-1°C-1Heat capacity of
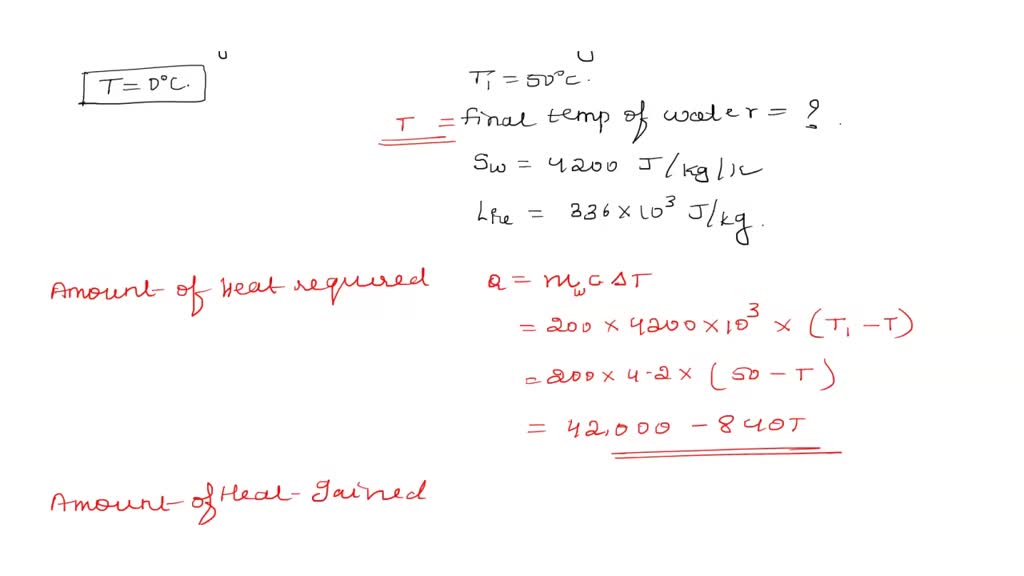
SOLVED: A piece of ice of mass 40 g is added to 200 g of water at 50oC. Calculate the final temperature of water when all the ice has melted. Specific heat

single serve dessert with 19 grams of quality protein 👇👇 🍫I put my
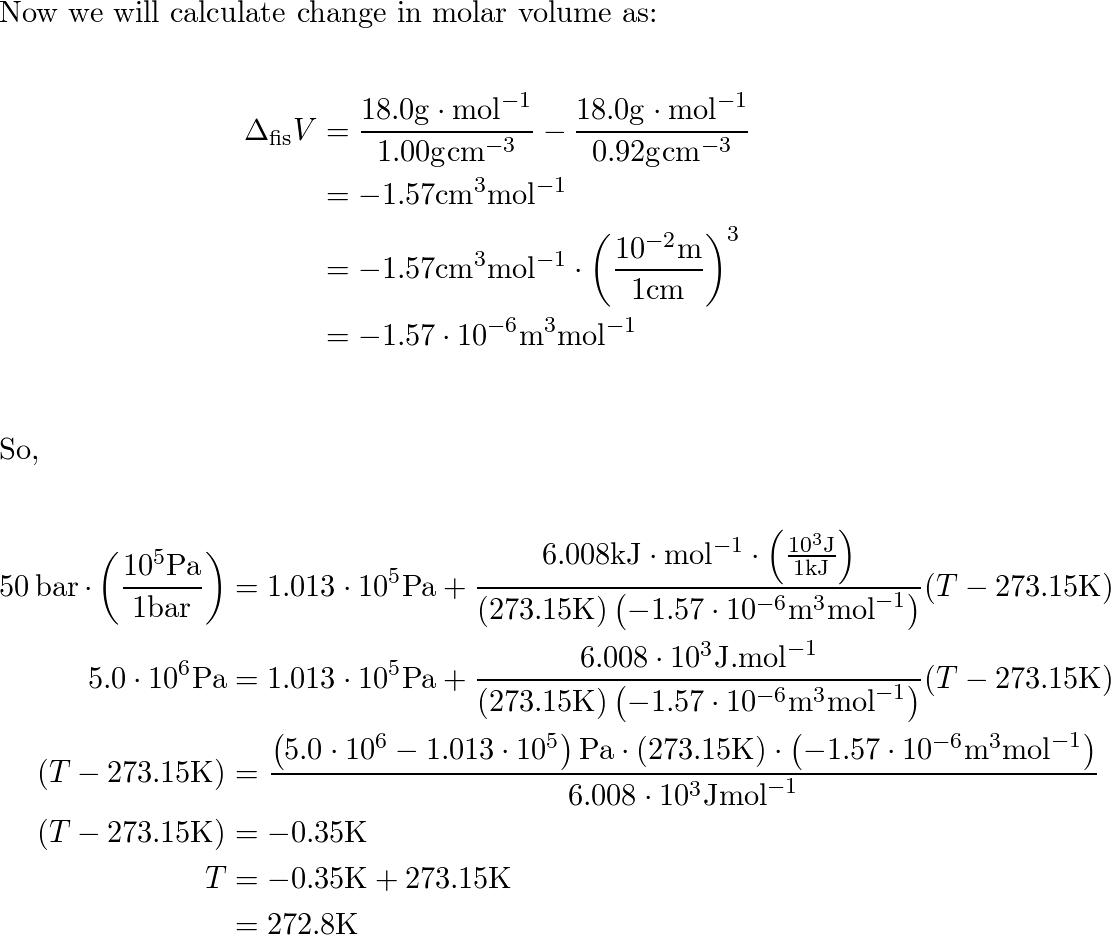
a) Estimate the melting point of ice under a pressure of 50

SOLVED: A cup of hot 50°C water is poured onto 35g of ice at 0°C until the entire system ends up at 20°C. How much energy is needed to melt the 35g

Chem dems - www-personal.edfac.usyd.edu.au

A 17.5 g sample of metal at 125.0°C is placed in a calorimeter with 15.0 g of water at 25.0°C. if the temperature of the water rises to 30.0°C, what is the

Calaméo - Guide d'achat 2021-2022 EN

Culinaire #2:7 (december 2013 by Culinaire Magazine - Issuu
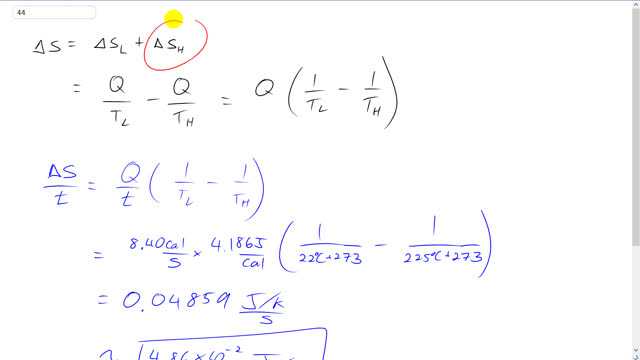
Giancoli 7th Edition, Chapter 15, Problem 44

8.2: Calorimetry (Problems) - Chemistry LibreTexts

First for Women January 16, 2023 (Digital)

Finding Final Temperature When Ice is Added to Water

Finding Final Temperature When Ice is Added to Water