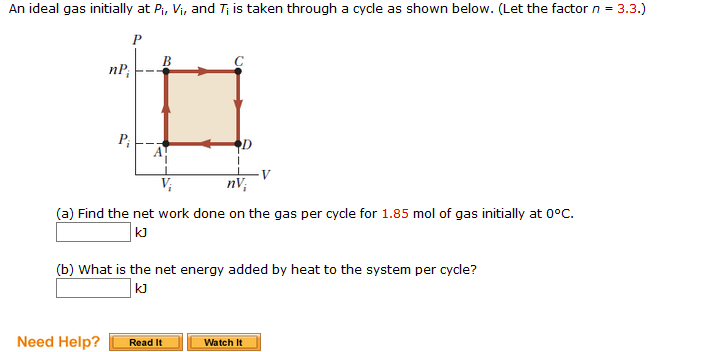
Solved An ideal gas initially at Pi, Vi, and Ti is taken

A 1.00 mol sample of monoatomic ideal gas is take through the cycle shown. At point A, the pressure, volume and temperature are P_i, V_i and T_i respectively. In terms of R

First Alert Radon Gas Test Kit, RD1

Van der Waals equation - Wikipedia

An ideal gas initially P_i ,V_i , and T_i is taken through a cycle as shown in Figure. (a) Find the net work done on the gas per cycle 1.00 mol of

An ideal gas initially P_i ,V_i , and T_i is taken through a cycle as shown in Figure. (a) Find the net work done on the gas per cycle 1.00 mol of
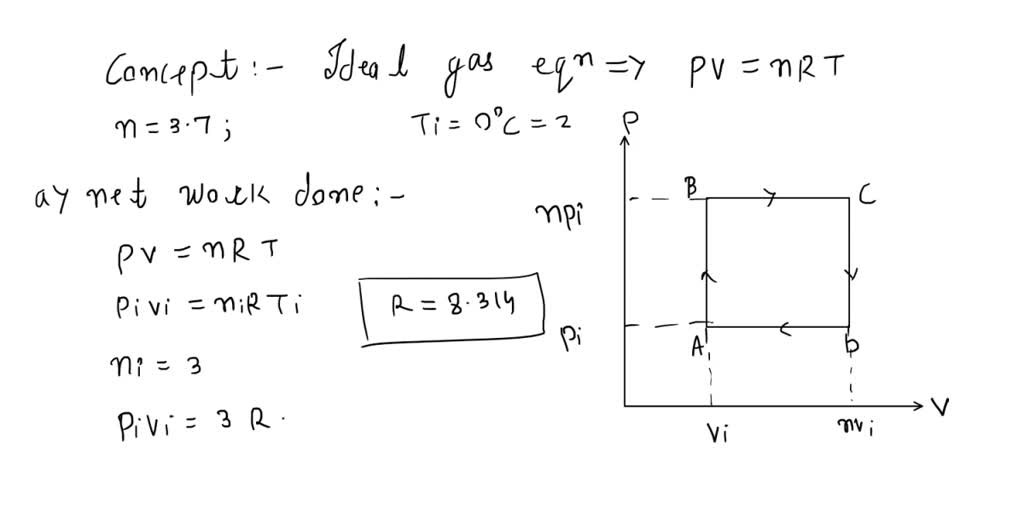
SOLVED: ideal gas initially at Pi, Vi, and Ti is taken through cycle as shown below: (Let the factor n 3.7.) nf Find the net work done on the gas per cycle
Let's Derive the Ideal Gas Law from Scratch!

Keyhole fluctuation and pore formation mechanisms during laser powder bed fusion additive manufacturing
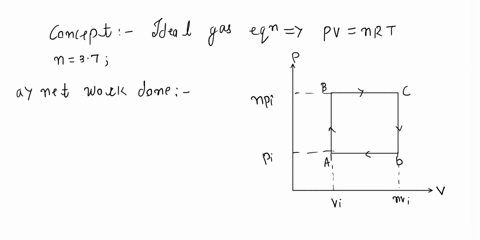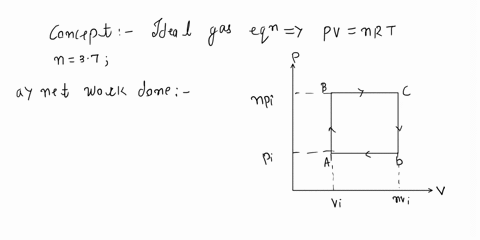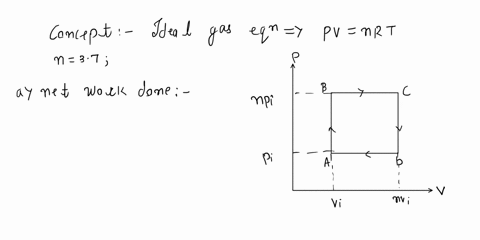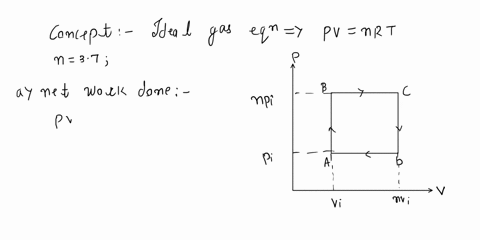
SOLVED: 'An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown below: (Let the factor n = 2.9.) (a) Find the net work done on the
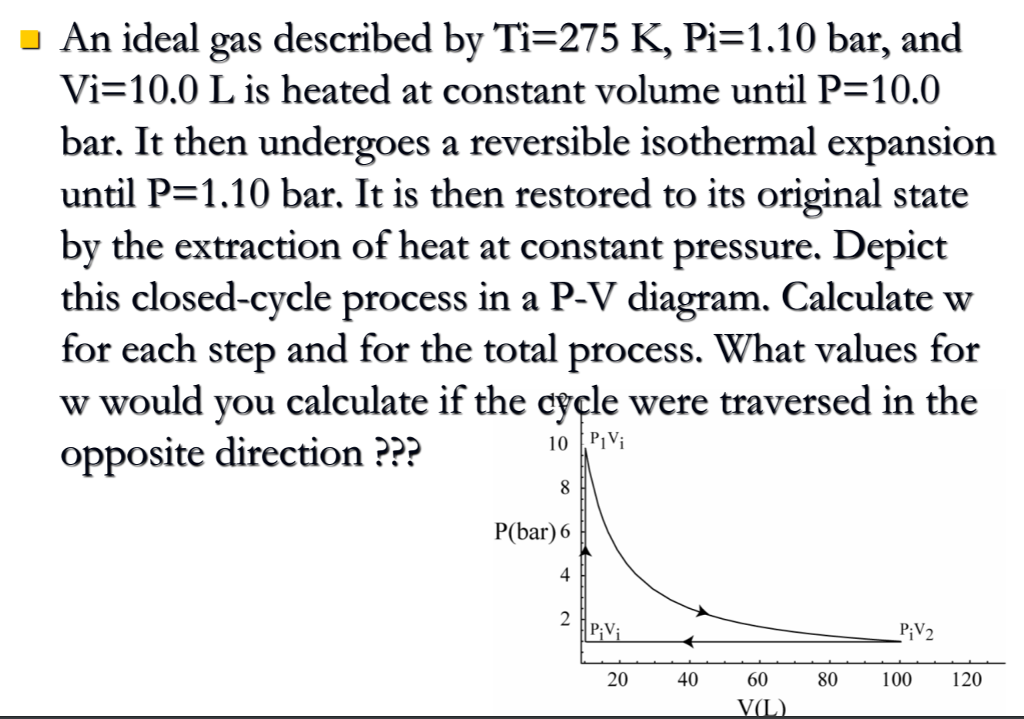
Solved - An ideal gas described by Ti-275 K, Pi-1.10 bar
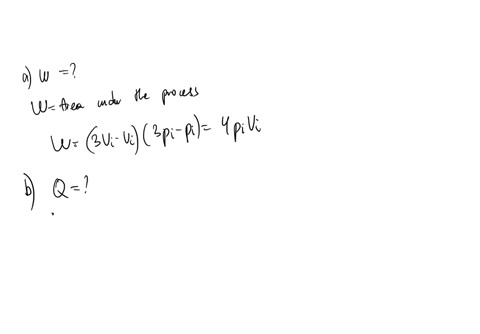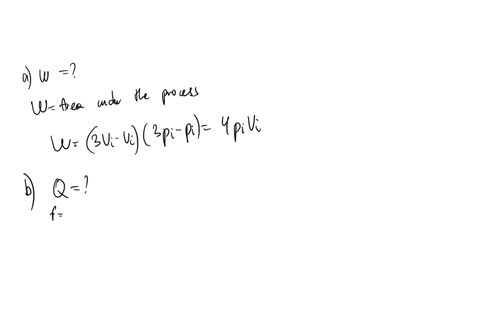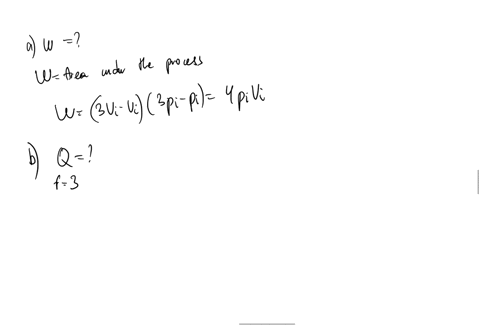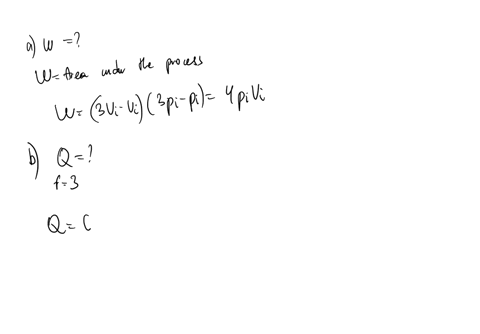
SOLVED: An ideal gas initially at Pi' Vi' and Ti is taken through a cycle as shown below. (Let the factor n 2.8.) nP; nV; a) Find the net work done on
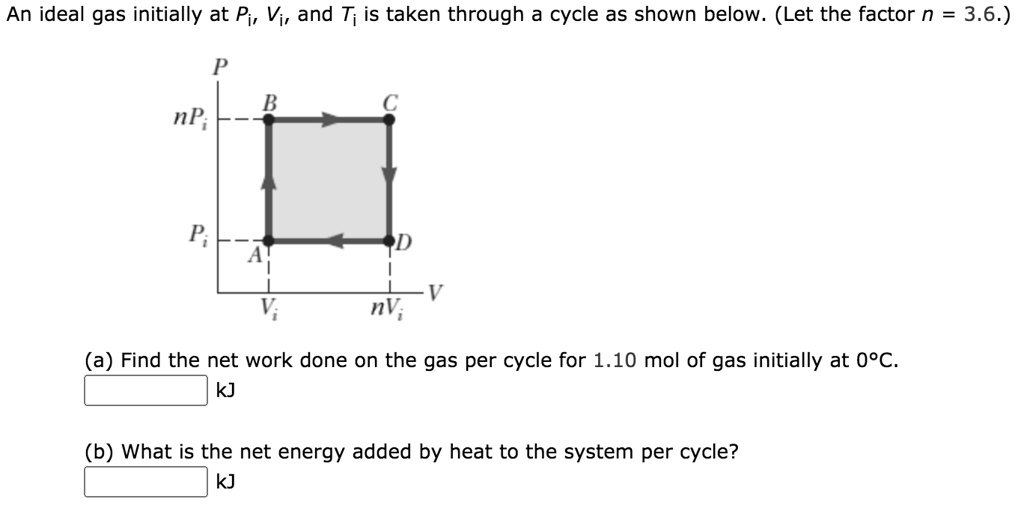
SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown below. (Let the factor n 3.6.) nP; P; nV; (a Find the net work done

Solved An ideal gas initially at Pi, Vi, and T is taken