Five Common Mistakes Submitting a Premarket Notification

How you can avoid the most common errors made when submitting a 510(k), the “premarket notification,” with simple measures
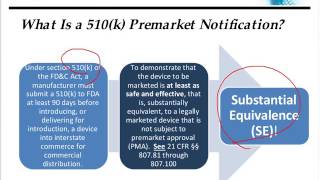
How to Prepare a 510(k) Quality Submission

Robert A. Allen, PhD on LinkedIn: #biocompatibility #meddevice #medicaldevice #medicaldevices…

The Top 10 Most Significant Changes Introduced by the New EU MDR (and how to avoid the common mistakes)

Examining the HHS Proposal for Premarket Notification Exemptions

Top 5 Best Premarket Stock Screeners for 2024 - StocksToTrade

Inclusion of PRO in summary documents, separated by endpoint

PPT - Premarket Processes & Pathways to Market Pre-amendment, Exempt, 510(k), and 513(g) PowerPoint Presentation - ID:1605402

FDA 510k Premarket Notification: Essential Requirements

5 Tips for Successful Medical Device Registration Across Global Markets