The 12-item short form health survey (SF-12)

Download scientific diagram | The 12-item short form health survey (SF-12) from publication: Measurement Properties of the SF-12 Health Survey in Parkinson's Disease | The 12-item Short-Form Health Survey (SF-12) is an abbreviated version of the SF-36, one of the most widely used patient-reported health outcome rating scales. Similar to the SF-36, it yields summary scores of physical and mental health (PCS and MCS, respectively). However, | Parkinson's Disease, Health Surveys and Health Status | ResearchGate, the professional network for scientists.

Mean SF-12 PCS (a) and MCS (b) scores during the 12-month follow-up
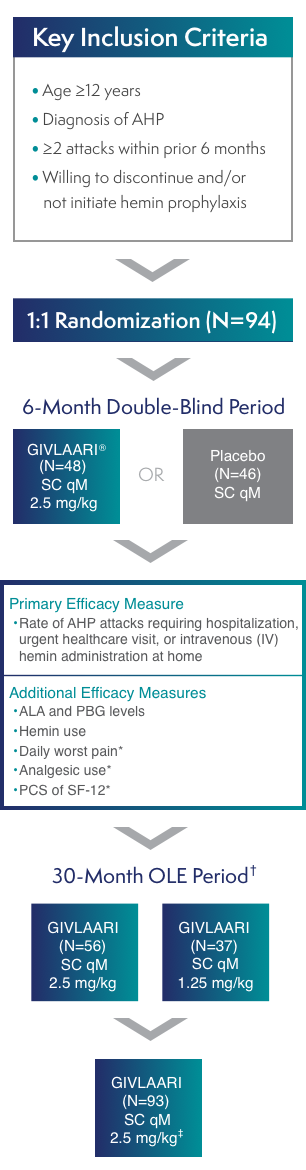
GIVLAARI® (givosiran) Efficacy & Safety

The 12-Item Short Form Health Survey (SF-12) physical component score

PDF) A 12-Item Short-Form Health Survey

Association between general and oral health-related quality of life in patients treated for oral cancer. - Abstract - Europe PMC

Annual high-dose vitamin D3 and mental well-being: randomised controlled trial, The British Journal of Psychiatry
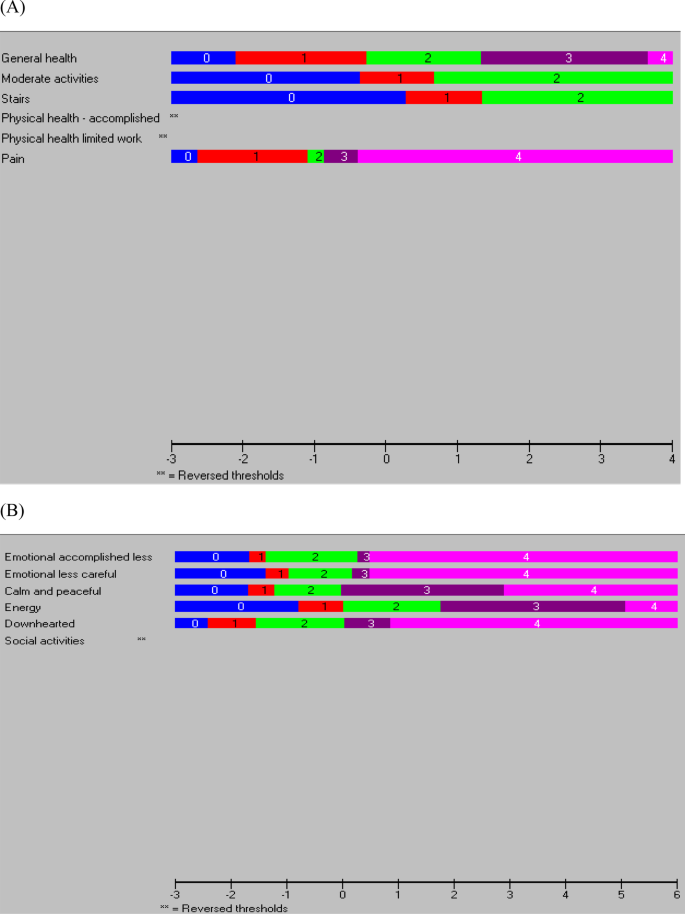
Measurement properties of the 12-item Short Form Health Survey version 2 in Australians with lung cancer: a Rasch analysis, Health and Quality of Life Outcomes

Beware of the origin of numbers: Standard scoring of the SF‐12 and SF‐36 summary measures distorts measurement and score interpretations - Hagell - 2017 - Research in Nursing & Health - Wiley Online Library

Mos Sf 12 General Health Survey - Fill and Sign Printable Template Online

SciELO - Brasil - Propriedades psicométricas do instrumento de avaliação da qualidade de vida: 12-item health survey (SF-12) Propriedades psicométricas do instrumento de avaliação da qualidade de vida: 12-item health survey (SF-12)
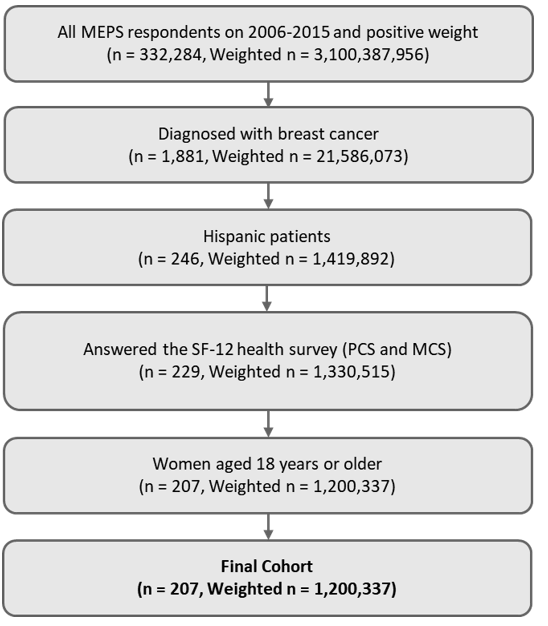
Association of Health-Related Quality of Life with Breast Cancer Survival among Hispanic Population Using 10 Years of MEPS National Sample Cohort Data, Clinical Oncology and Research, Science Repository
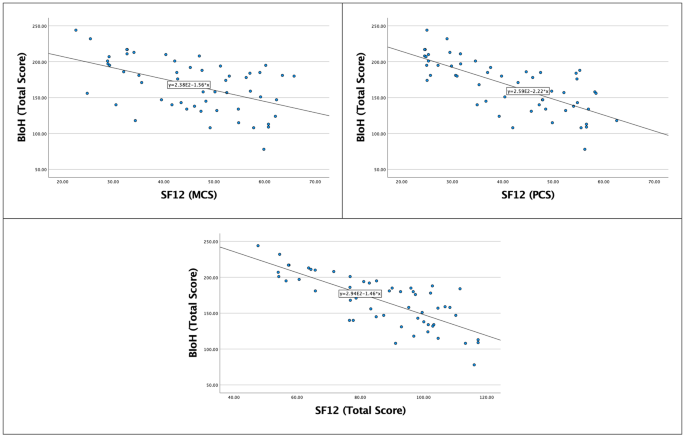
Arabic translation, cultural adaptation, and validation of the Bristol Impact of Hypermobility questionnaire, Journal of Patient-Reported Outcomes