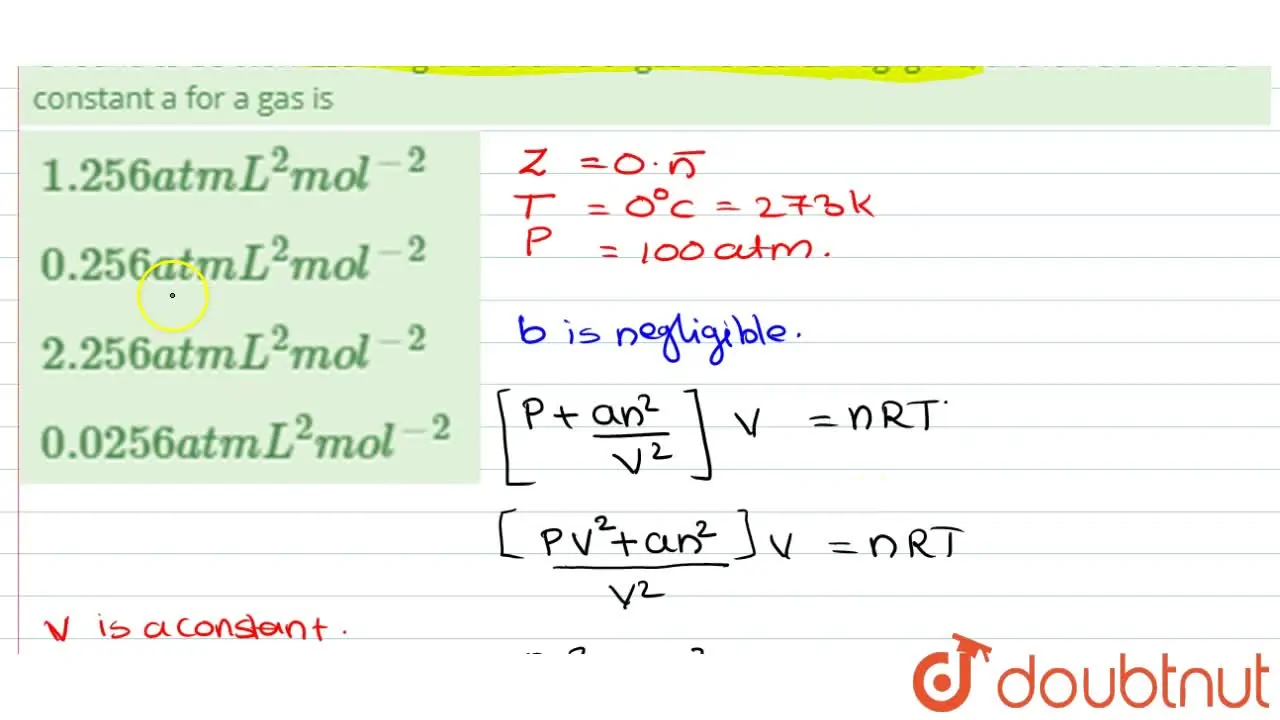
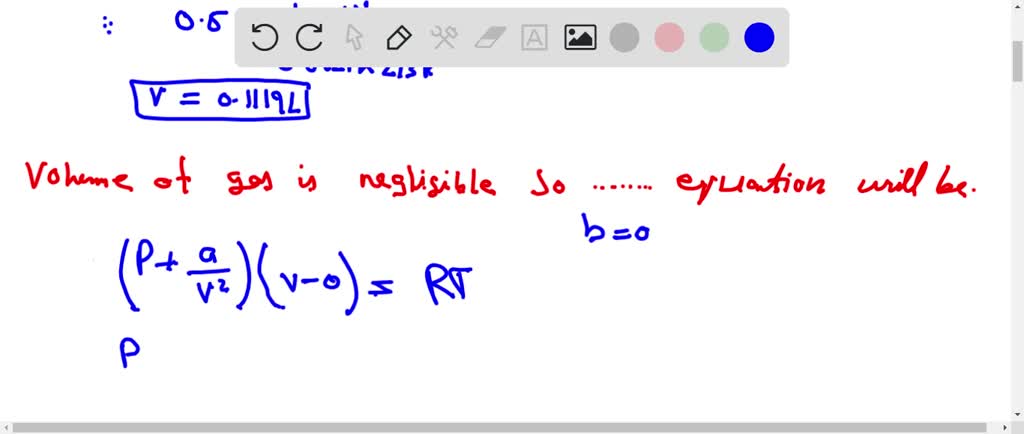
The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor compressibility factor for onemole of a van der waals gas at 0c
Click here👆to get an answer to your question ✍️ The compression factor -compressibility factor- one mole of a van der Waals gas 0-C and 100 atm pressure is found to be 0-5- Assuming that the volume of a gas molecule is negligible- calculate the van der Waals- constant a
The compressibility factor for one mol of a vanderwalls gas at 0 degree c and 100atm pressure is .5 then what will be the volume of 2 mols of this gas

The compression factor (compressibility factor) for one mole of a vander Waals gas at 0^∘C and
The compression factor (compressibility factor) for one mole of a van der Waals' gas at 0ºC and 100 atm pressure is - Sarthaks eConnect

Compressibility Chart - an overview

Djective lype Questions The compressibility factor of N2 moderate pressure range is equal to [where a & b are the van der Waal's constants] Pb Pb (2) 1- (1)RT RTV (3) (1RTV

Solved (Triple-Play Bonus) For a certain gas, the
Malayalam] The compressibility factor for definite amount of van der

16.4: The Law of Corresponding States - Chemistry LibreTexts
⏩SOLVED:The compression factor (compressibility factor) for one mole…