The correct order of increasing bond length of \( \mathrm{C
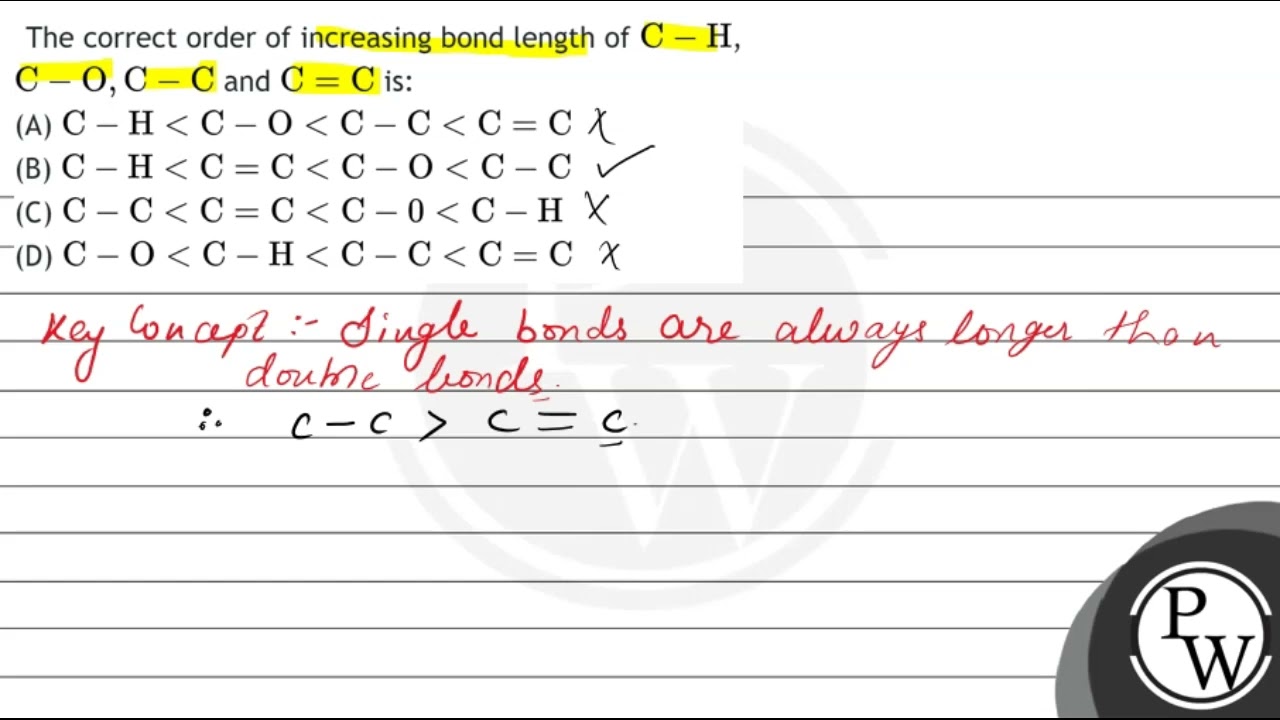
The correct order of increasing bond length of \( \mathrm{C}-\mathrm{H} \), \( \mathrm{C}-\mathrm{O}, \mathrm{C}-\mathrm{C} \) and \( \mathrm{C}=\mathrm{C} \


The correct order of increasing bond length of C - H ,C - O, C - C and C = C is
Which of the following has the longest bond length, C=O, N=O, C=C, or C=N? - Quora
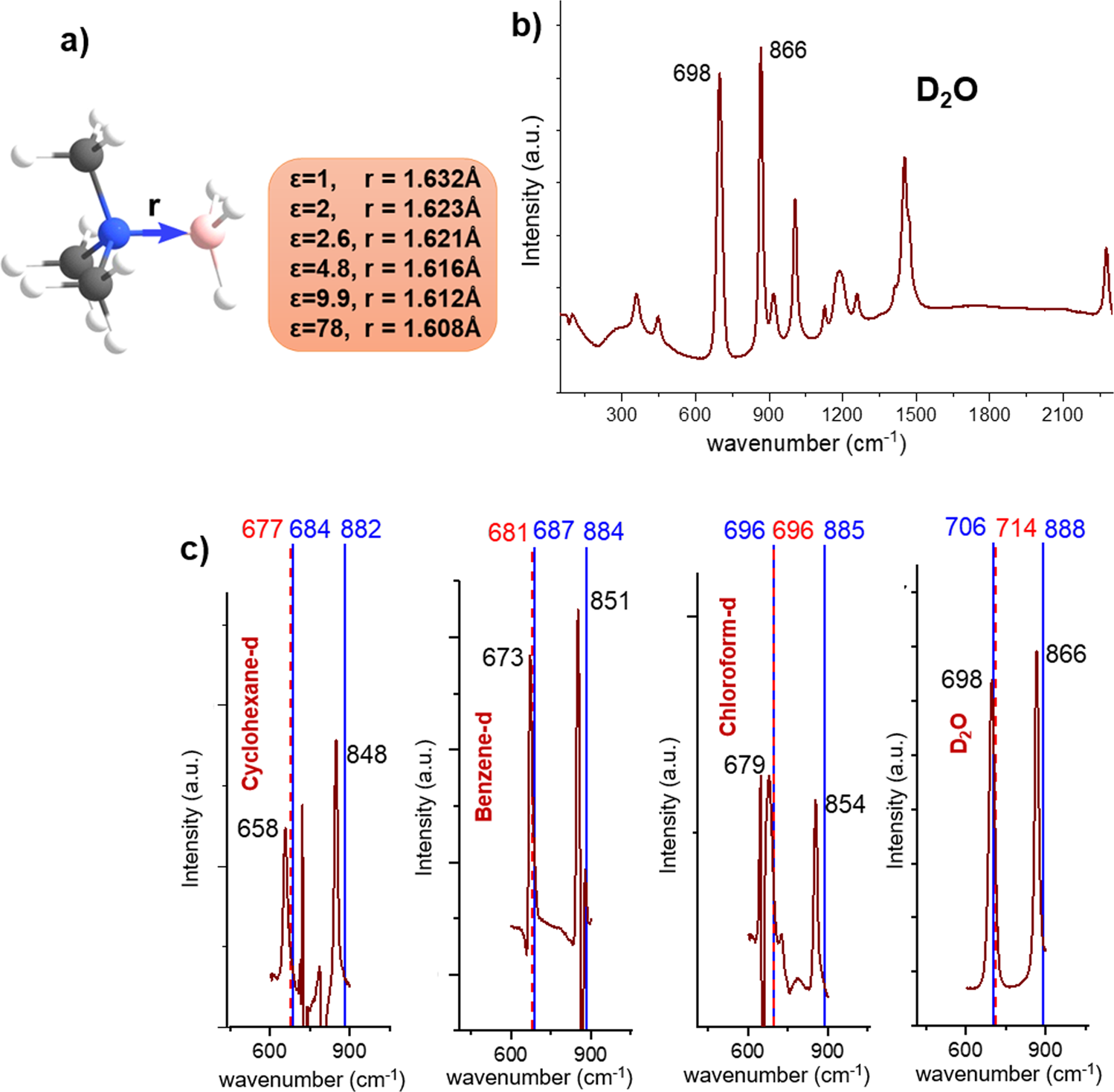
The stability of covalent dative bond significantly increases with increasing solvent polarity

Hypervalent molecule - Wikipedia


In the Molecular Orbital diagram of SF6 ,find the sulfur orbitals which are non-bonding: px, py, pz dxy, dxz, dyz dx2 ??y2 , dz2 S . Explain your answer.

Structural parameters including bond lengths (Å), divalent bond angles
which of the following is the correct order of C C bond lengths among these compounds—–1.CH3 O CH=CH NO2 2.CH2=CH CH3 3.CH2=CH NO2 4.CH2=CH2

The correct order of increasing bond length of C - H ,C - O, C - C and

The correct order of increasing bond length of C - H ,C - O, C - C and