Useful Forms – UK NEQAS – ICC & ISH

PDF) Best Practice No 176: Updated recommendations for HER2 testing in the UK
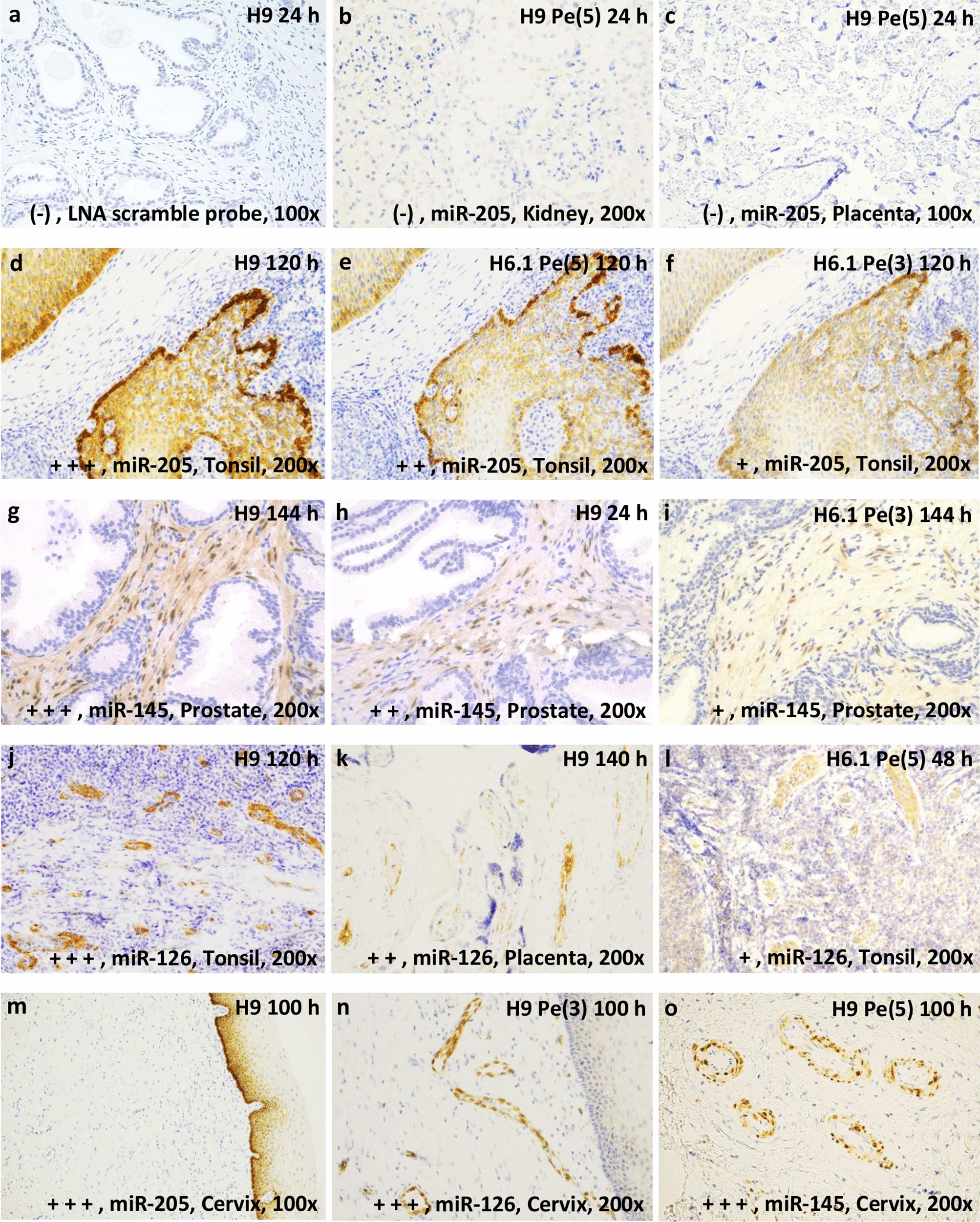
A novel approach for microRNA in situ hybridization using locked nucleic acid probes

Immunohistochemistry Review Of The Technical Test - NordiQC

PDF) Quality assurance guidance for scoring and reporting for pathologists and laboratories undertaking clinical trial work: Quality assurance in clinical trials

Quality Control of Immunohistochemical and In Situ Hybridization Predictive Biomarkers for Patient Treatment: Experience from International Guidelines and International Quality Control Schemes

ERBB2 mutation is associated with sustained tumor cell proliferation after short-term preoperative endocrine therapy in early lobular breast cancer - Modern Pathology

Accreditation and Scope – UK NEQAS – ICC & ISH

We are a leading provider of AI-driven precision pathology software for research and diagnostics

UK NEQAS IIA - Changing Your Method Details

Useful Forms – UK NEQAS – ICC & ISH

Collaboration between Visiopharm and Grundium advances AI-driven pathology solution for labs - Visiopharm

UK NEQAS IQN Path

NPIC and Visiopharm collaborate to advance H&E staining standardisation - Visiopharm
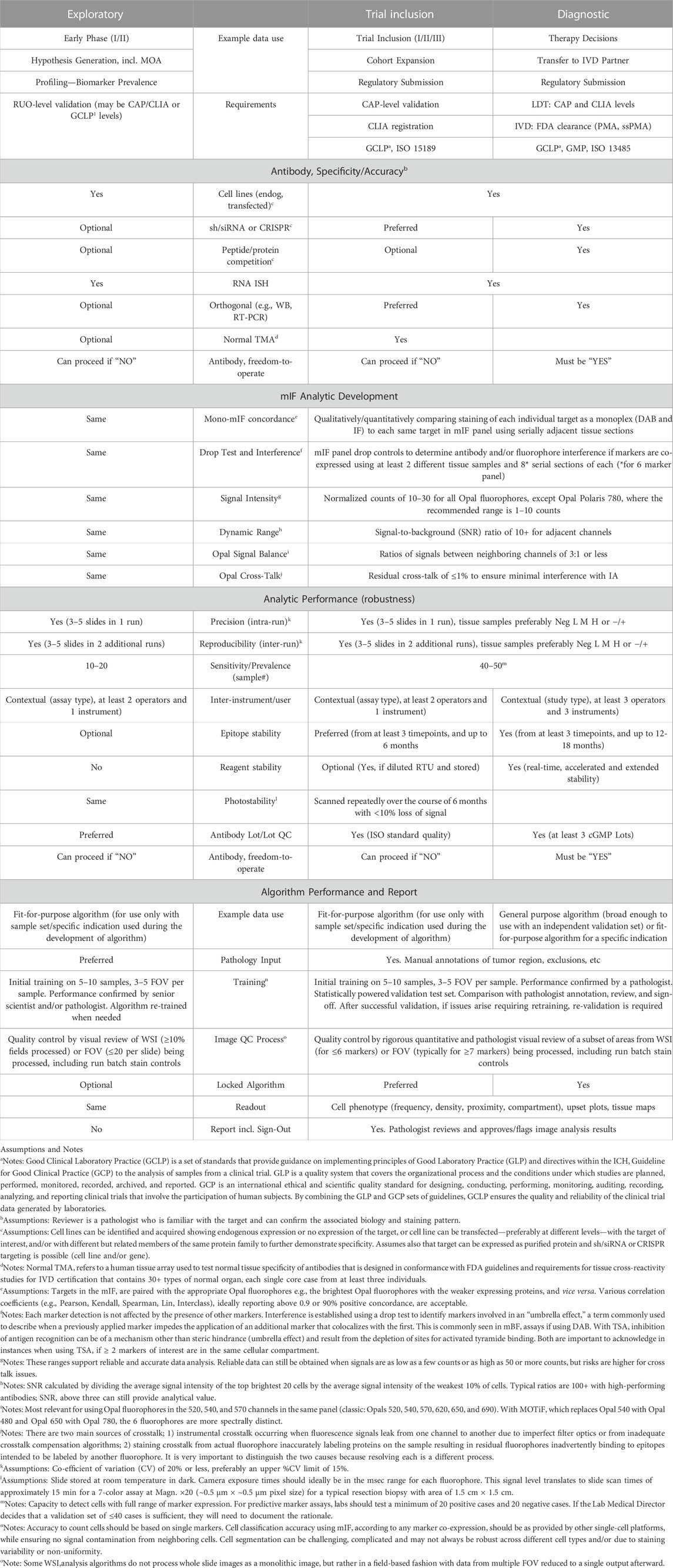
Frontiers Companion diagnostic requirements for spatial biology using multiplex immunofluorescence and multispectral imaging

e-Journal – UK NEQAS – ICC & ISH