Write the expression for the compressibility factor (Z) for one mole of a gas. Write the value of Z for an


Gas Compressibility - an overview

PPT - GASES PowerPoint Presentation, free download - ID:2088317

Physical Chemistry The Compression Factor (Z) [w/1 example]
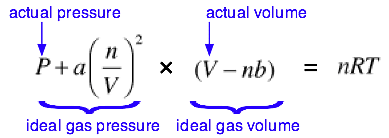
Derivation of Van Der Waals Equation: Real & One Mole of Gas

cdn.kastatic.org/ka-perseus-images/854dcada2b7466c

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks
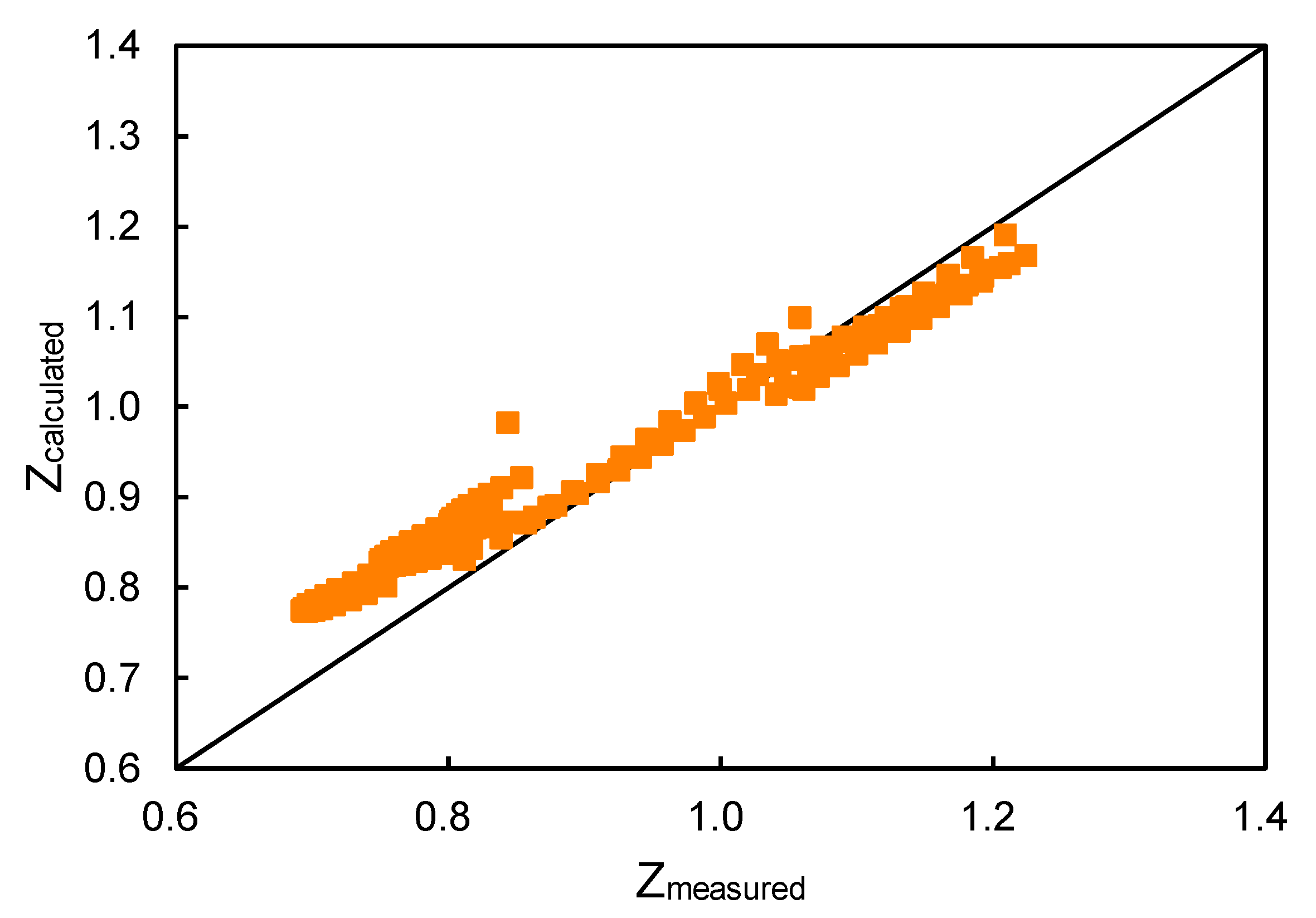
Energies, Free Full-Text

Van der Waals equation, when pressure correction is ignored, one mole can be written as P(V - b) = RT. The correct expression compressibility factor will be

The compressibility factor (Z) of one mole of a van der Waals' gas of negligible 'a ' value is:1dfrac{bp}{RT}1+dfrac{bp}{RT}1-dfrac{bp}{RT}

Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of
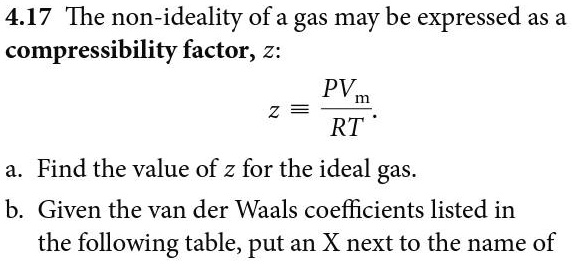
SOLVED: 4.17 The non-ideality of a gas may be expressed as a compressibility factor, z: PVm RT a. Find the value of z for the ideal gas. b. Given the van der

Deviation from ideal gas behaviour

SOLVED: Derive the mathematical expression expressing the compressibility factor Z of a real gas depending on the reduced variables; Explain in detail how the volume of the actual gas at a given

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application