The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n

The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n A.For the gas A, a=0 and its dependence
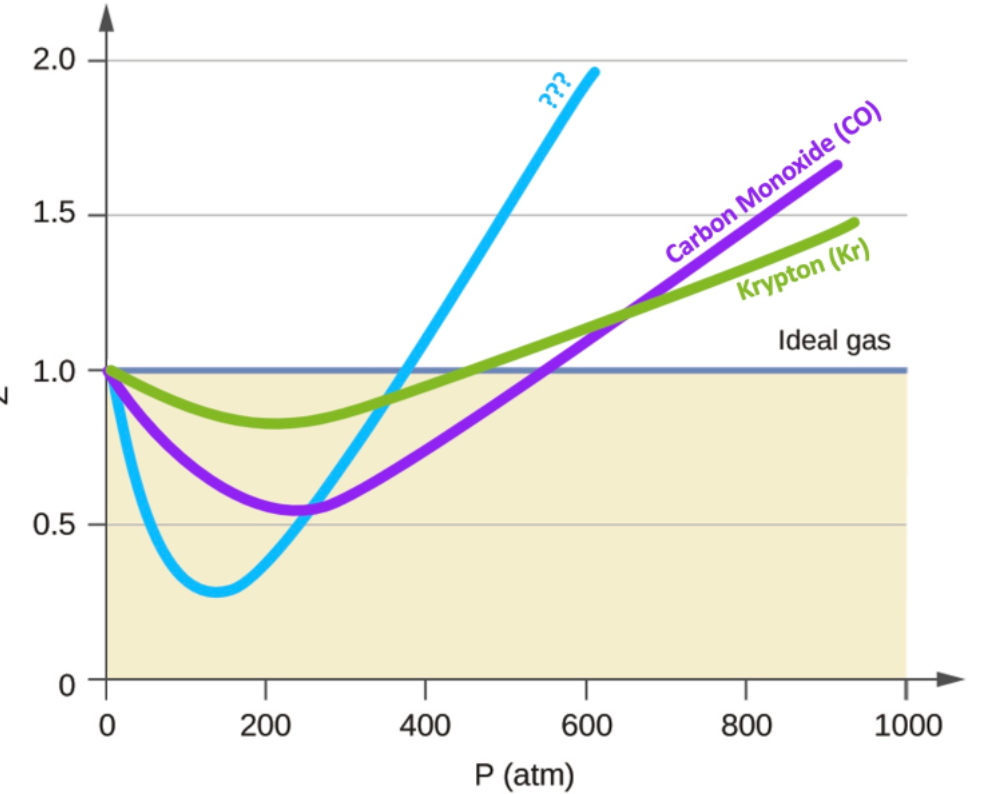
Solved Below is a plot of the compressibility factor (Z) as

Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with

The graph of compressibility factor (Z) :vs: P one mole of a real

The given graph represents the variation of Z(compressibility factor =- PV nRT ) versus P, three real gases A, B and C. Identify the only incorrect statement. Ideal gas P (atm) (A)

Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with

The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `

stackoverflowresearcher/archimedes.txt at master · MichalPaszkiewicz/stackoverflowresearcher · GitHub

The given graph in Fig represents the variation of Z compressibility f actor = pV / nRT vs.P, for the three real gases A , B and C.identify the only incorrect statement
stackoverflowresearcher/archimedes.txt at master · MichalPaszkiewicz/stackoverflowresearcher · GitHub

Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with

The given graph represents the variation of Z(compressibility factor =displaystyle frac{mathrm{P}mathrm{V}}{mathrm{n}mathrm{R}mathrm{T}}) versus mathrm{P}, three real gases mathrm{A}, mathrm{B} and C. Identify the only incorrect statement.For the gas C
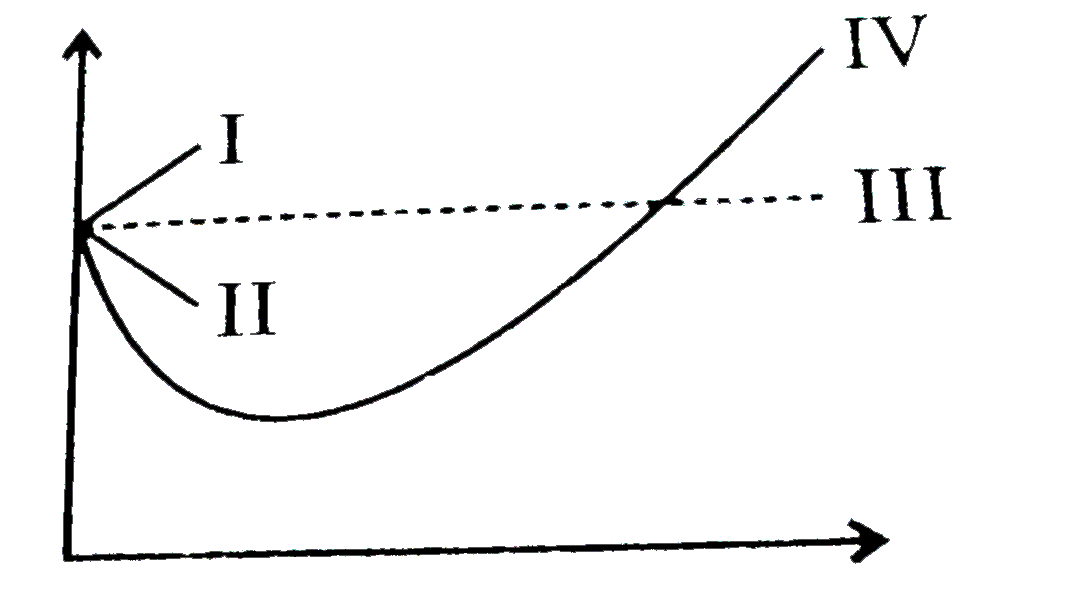
Telugu] The variation of compressibility factor (Z) with pressure (p

The given graph represents the variation of Z(compressibility factor =displaystyle frac{mathrm{P}mathrm{V}}{mathrm{n}mathrm{R}mathrm{T}}) versus mathrm{P}, three real gases mathrm{A}, mathrm{B} and C. Identify the only incorrect statement.For the gas C

The given graph represent the variations of Z (compressibility factor (Z)= dfrac {pV}{nRT}) versus P, three real gases A, B and C. Identify the only incorrect statement.For the gas B, b=0 and its

variations of 2 12.7 (a) eb (c)-(ar (d) - 6. The given graph represent the variations (compressibility factor (Z)=- gases A, B and C. Identify the only incorrect statement pl) versus p