Why do pressure and temperature increase during the compression of

The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Learn more about this in this article.

Lesson 9 COMPRESSION PROCESSES Apply the ideal gas laws to SOLVE

What is meant by dissipation of energy? - tec-science
Why does the temperature of a gas or a liquid increase when you

Boyle's law, Definition, Equation, & Facts
Evaluation of In-Die Compression Data for a Deeper Understanding

Relationship Between Pressure and Temperature

Air at 100 KPa and 280 K is compressed steadily to 600 KPa and 400
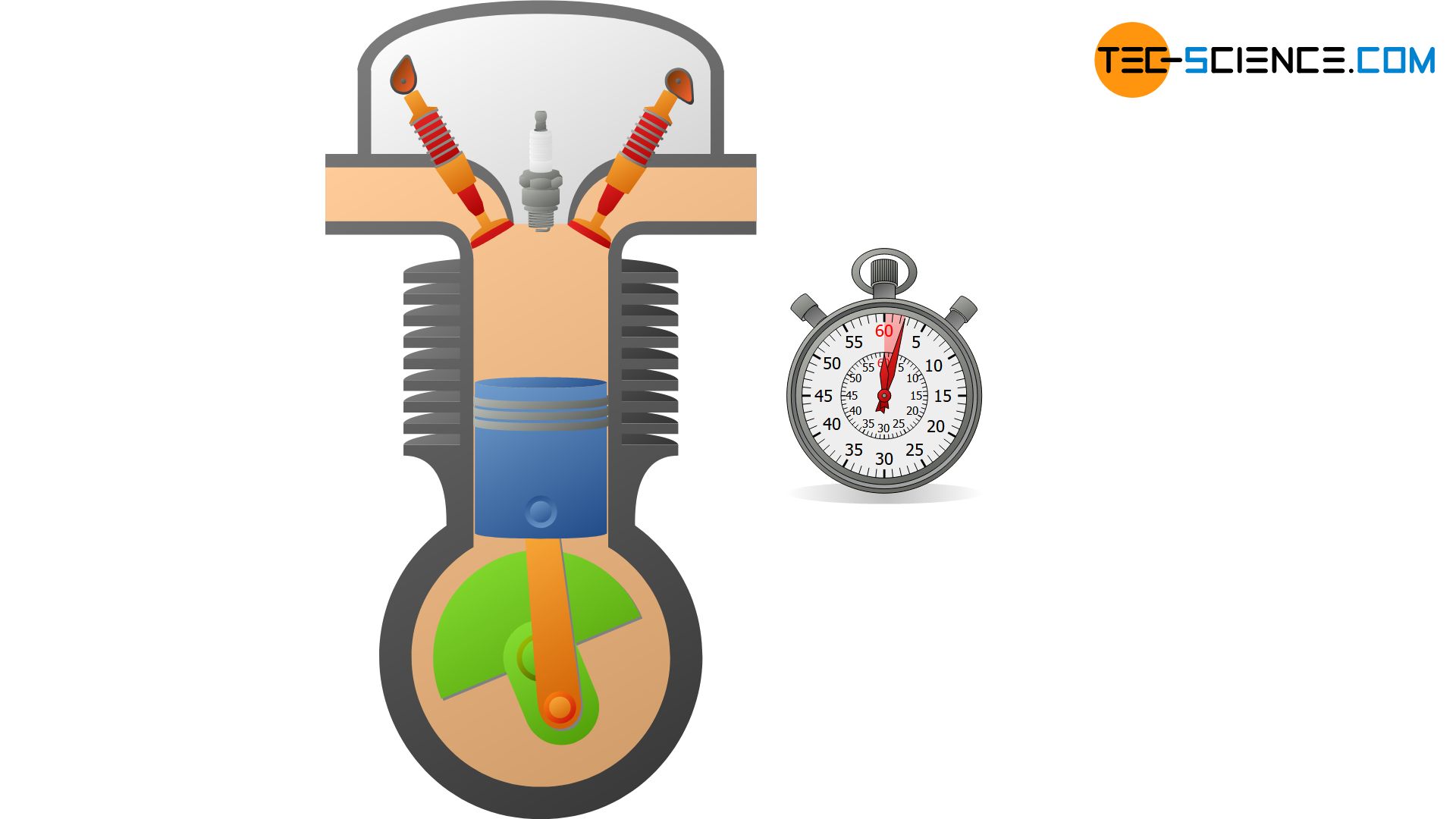
Isentropic (adiabatic) process in a closed system - tec-science
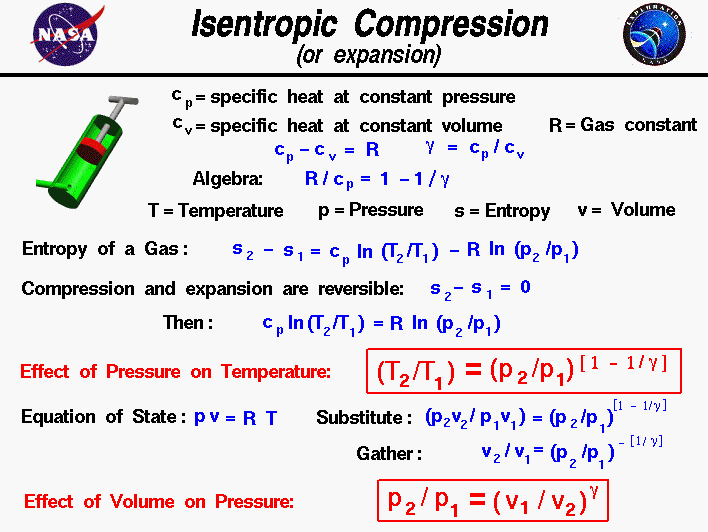
Isentrophic Compression, Glenn Research Center

The Top 10 FAQs About Compressed Air - Fun Facts About Compressed Air
A gas is suddenly compressed to 1/4th of its original volume.what
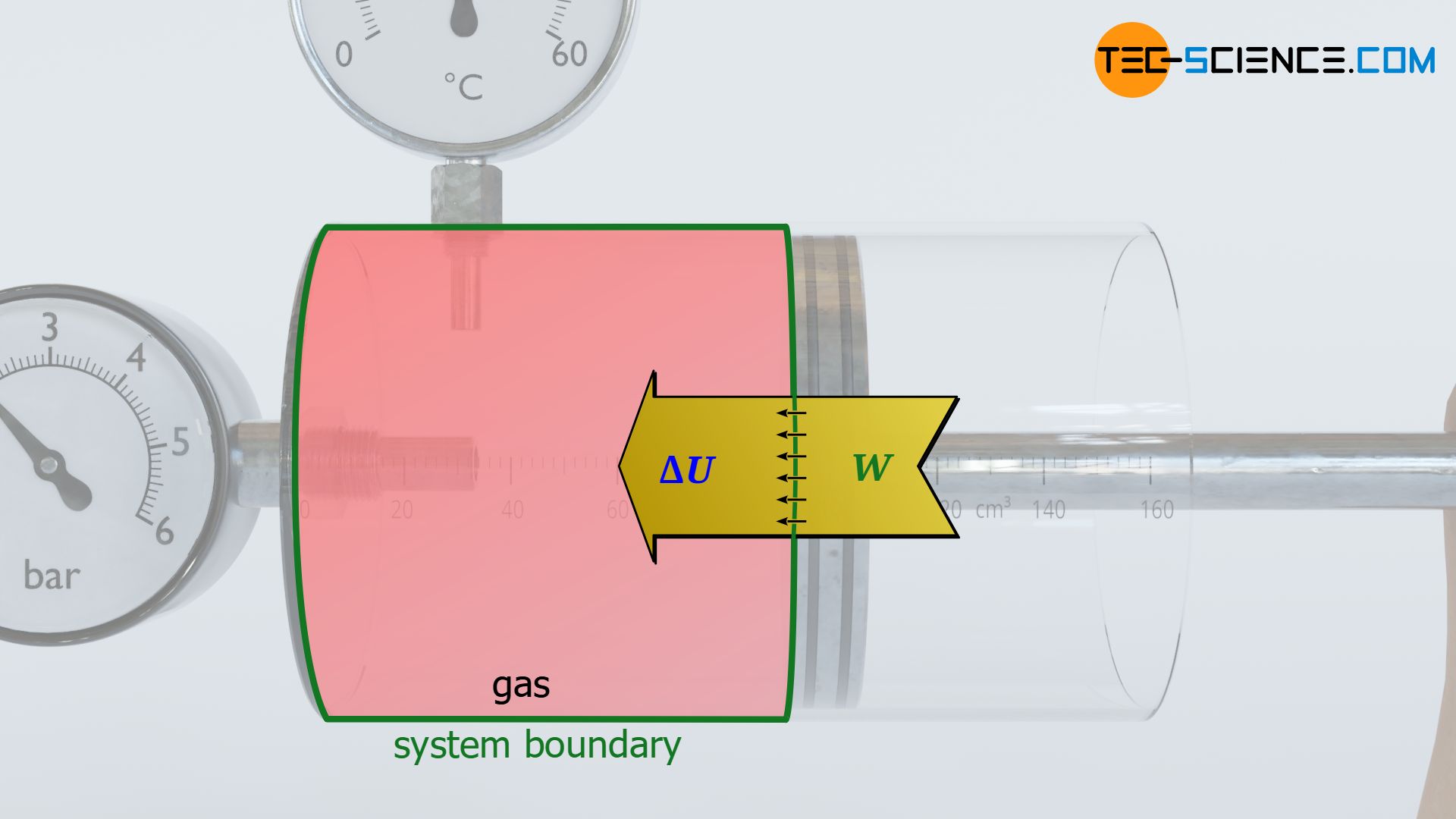
What is meant by dissipation of energy? - tec-science
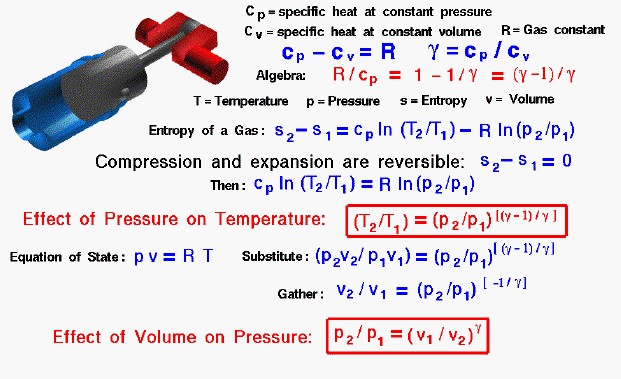
Compression and Expansion, Glenn Research Center

8.2: Relating Pressure, Volume, Amount, and Temperature: The Ideal