Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt


The compressibility factor of a gas is defined as Z=PV/nRT. The compressibility factor of an ideal gas is:1-1zeroinfinite

Compressibility factor - Wikipedia

Compressibility factor, Z of a gas is given as Z = pV / nRTi What is the value of Z for an ideal gas?ii For real gas what will be the effect
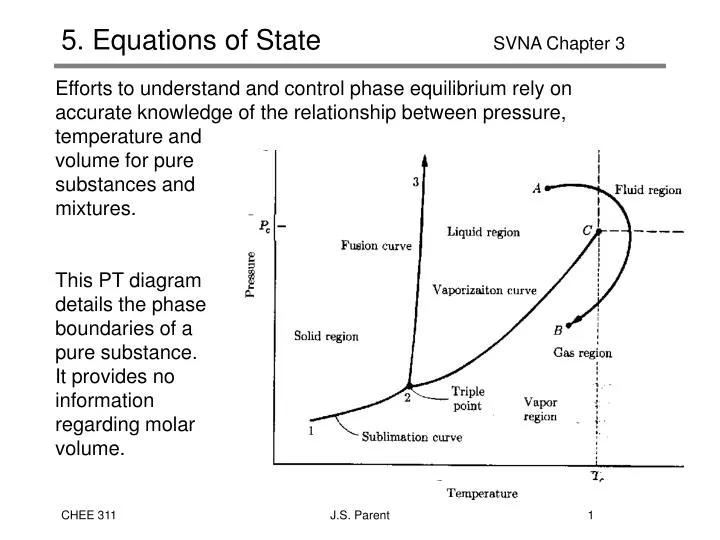
PPT - 5. Equations of State SVNA Chapter 3 PowerPoint Presentation, free download - ID:5380992

Properties of Gas Manik

ANSWERED] Q 32 Compressibility factor Z of a gas is given as Z pV nRT - Kunduz

Gas compressibility factor Z: Ideal gas vs Real gas
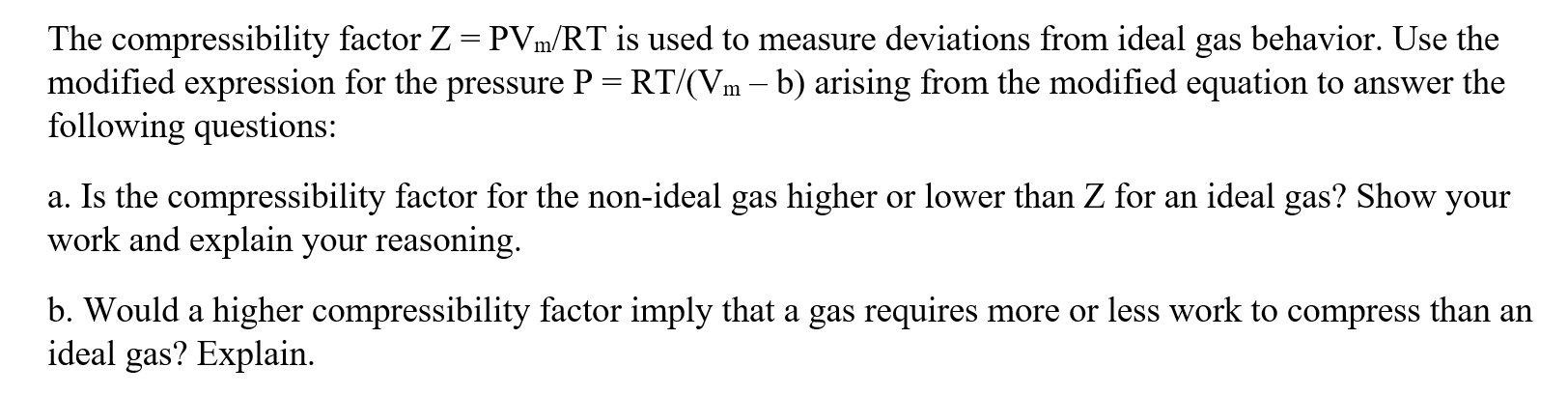
Solved The compressibility factor Z = PVm/RT is used to

Gas compressibility factor Z: Ideal gas vs Real gas

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt
What does a compressibility factor >1 signify, apart from a deviation from the ideal gas behaviour? Is it more compressible? - Quora

Ideal Gases & Real Gases, PDF, Gases