What is the compressibility factor (Z) for 0.02 mole of a van der Waal

(d) (0.1+(1000xx(0.02)^(2))/(V^(2)))V=20xx0.02 =0.1V^(2)-0.4V+0.4=0 =V^(2)-4V+4=0 implies" "V=2L Z=(PV)/(nRT)=(0.1xx2)/(20xx0.02)=0.5

0.585%NaCl solution at 27∘C has osmotic pressure of

Filo Student Questions For CBSE , Grade 9

Compressibility factor (gases) - Citizendium

11111 Umu) 32 min 46. The ratio of van der Waals' constants a and

Physical Chemistry The Compression Factor (Z) [w/1 example

DPP No. : 21 Total Marks : 40 Max. Time : 10mln Single choice Objective (..
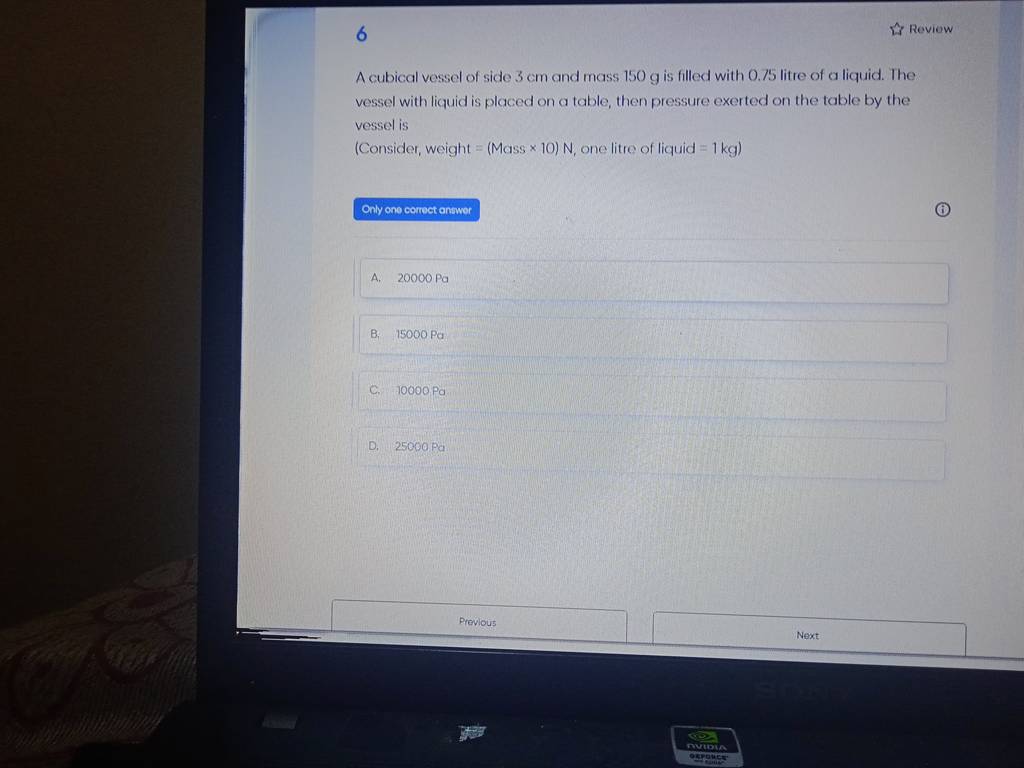
Filo Student Questions For CBSE , Grade 9

Compressibility factor - Wikipedia

What is the compressibility factor (Z) for 0.02 mole of a van der

SOLVED: The van der Waals constants for SO2 are a = 6.775 atm L^2
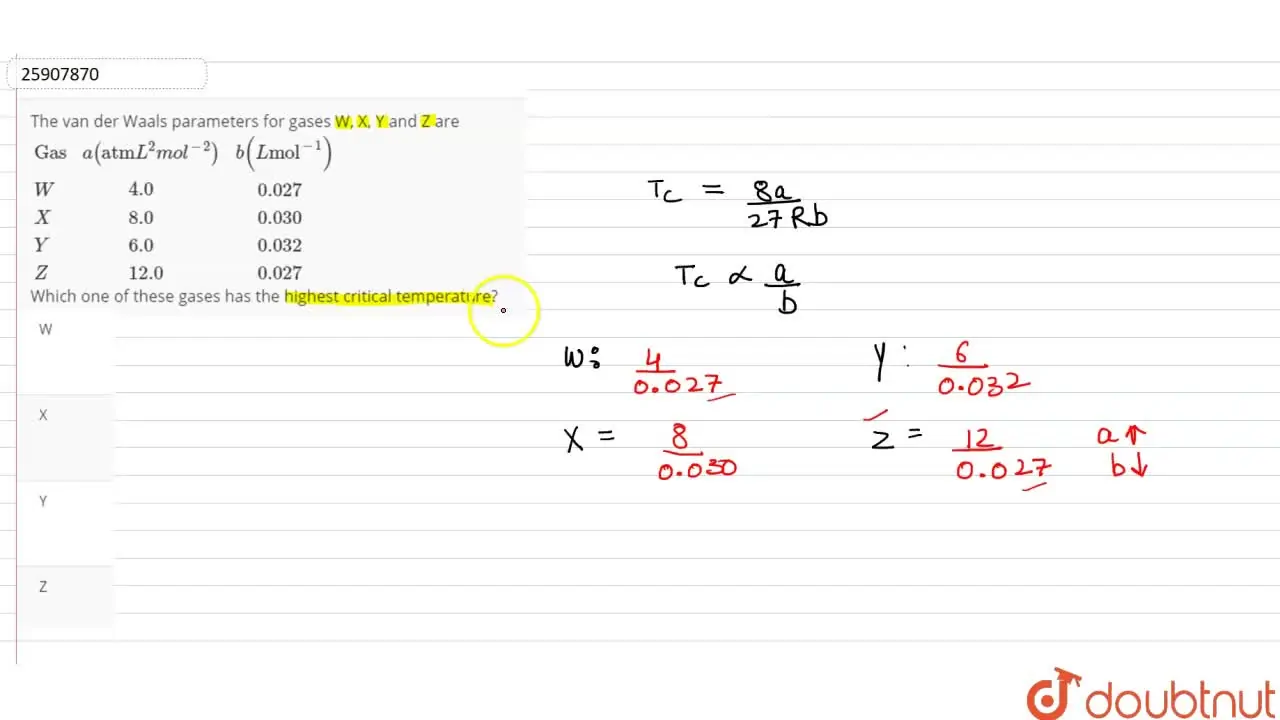
The van der Waals parameters for gases W, X, Y and Z are {:(Gas,a(