Solved] Why is the compressibility factor less than 1 at most conditions?

Gas compressibility factor Z: Ideal gas vs Real gas
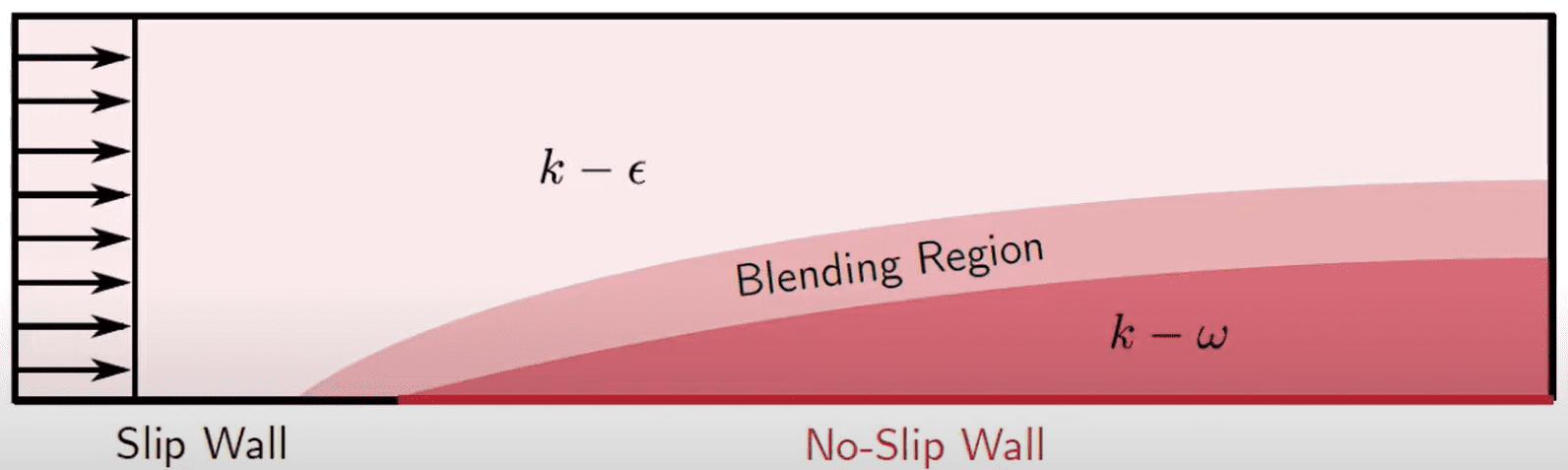
K-Omega Turbulence Models, Global Settings
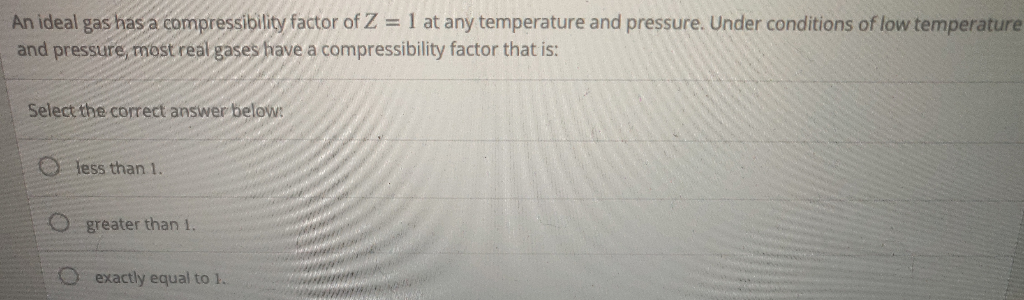
Solved An ideal gas has a compressibility factor of Z = 1 at

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Compressibility factor (z): real gases deviate from ideal behav-Turito
What does a compressibility factor >1 signify, apart from a deviation from the ideal gas behaviour? Is it more compressible? - Quora

Assertion: Compressibility factor `(Z)` for non ideal gases is always greater than `1`.

Compressibility factor - Wikipedia
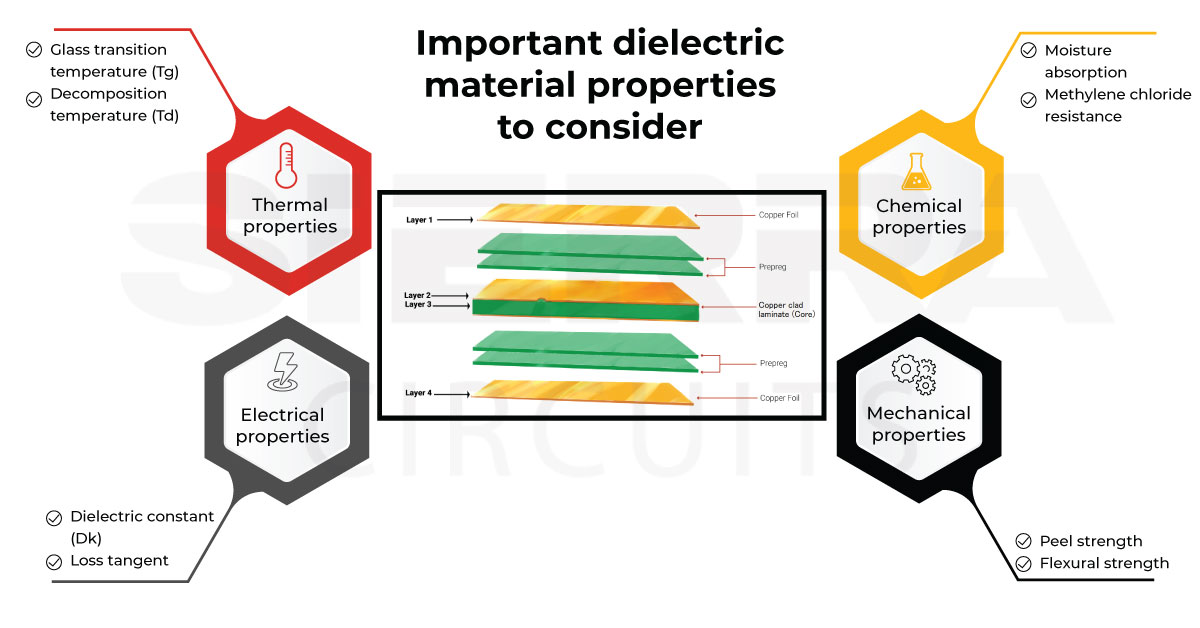
PCB Substrates: Knowing PCB Dielectric Materials

where Z is the compressibility factor that

Compressibility factor - Wikipedia
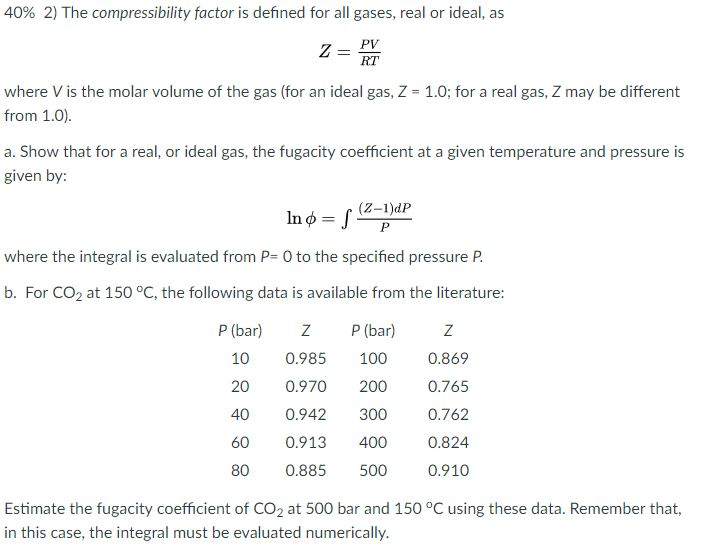
Solved 40% 2) The compressibility factor is defined for all