The given graph represent the variations of compressibility factor (z) = pV/nRT versus p, - Sarthaks eConnect
The given graph represent the variations of compressibility factor (z) = pV/nRT versus p, for three , the slope is positive for all real gases.

Given graph gives variation of dfrac{PV}{T} with P 1 gm of oxygen

Given graph gives variation of dfrac{PV}{T} with P 1 gm of oxygen

Yucation The given graph represent the variations of Z

For a given gas, a graph is shown between compressibility factor (Z) and Pressure (P).Select the incorrect statement(s) about the various temperature relations.a)Temperature T1 must be above critical temperature (TC).b)Temperature T2 may

DV (a) nb (c) – (n'a/v2) (d) - nb The given graph represent the
The given graph represents the variations of compressibility
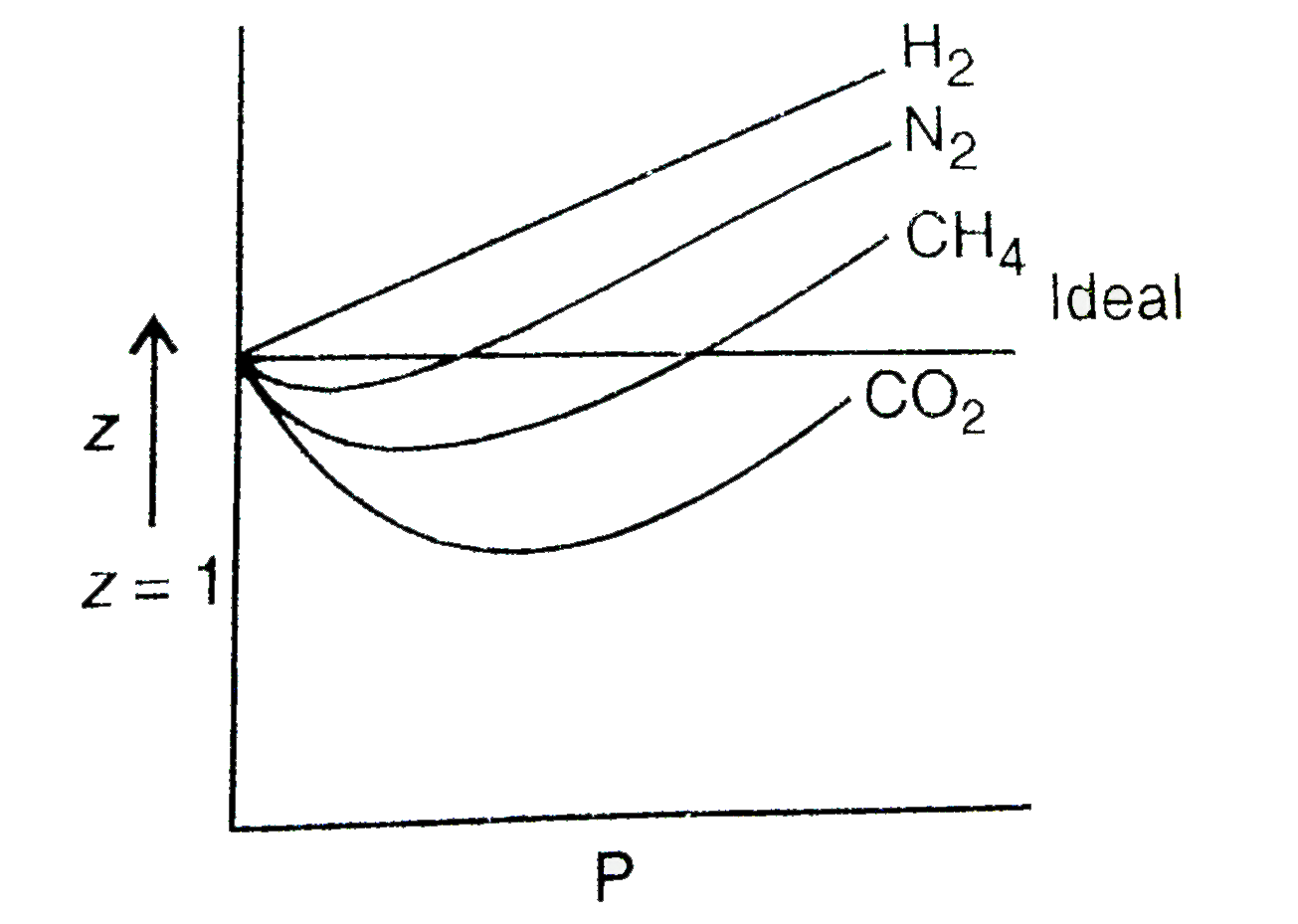
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

The given represents the variation of Z (compressibility factor

plotting - How to plot Compressibility factor Z vs Pressure P

Compressibility factor Z = PV / nRT is plotted against pressure as

For the gas C which is a typical real gas for which neither a nor b =0

The given graph represents the variation of Z(compressibility

The compressibility factor `(Z=PV//nRT)` for `N_(2)` at `223 K
Deduce the relation pv = nRT where R is a constant called