Two closed bulbs of equal volume V containing an ideal gas initially at pressure P i and temperature T 1 are connected through a narrow tube of negligible volume as shown in

Two closed bulbs of equal volume V containing an ideal gas initially at pressure P i and temperature T 1 are connected through a narrow tube of negligible volume as shown in the figure below. The temperature of one of the bulbs is then raised to T 2. The final pressure Pf is :P i T 1 T 2/ T 1+ T 2B. 2 P i T 1/ T 1+ T 2C. 2 P i T 1 T 2/ T 1+ T 2D. 2 P i T 2/ T 1+ T 2
Two closed bulbs of equal volume V containing an ideal gas initially at pressure P i and temperature T 1 are connected through a narrow tube of negligible volume as shown in the figure below- The temperature of one of the bulbs is then raised to T 2- The final pressure Pf is -P i T 1 T 2- T 1- T 2B- 2 P i T 1- T 1- T 2C- 2 P i T 1 T 2- T 1- T 2D- 2 P i T 2- T 1- T 2
The correct option is D 2P_i ( T_2T_1+T_2 )Since the above system is a closed one, the total number of moles of the ideal gas will be equal before and after th

⏩SOLVED:Two evacuated bulbs of equal volume are connected by a tube…
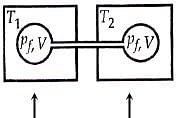
Two closed bulbs of equal volume (V) containing an ideal gas

⏩SOLVED:Two closed bulbs of equal volume ( V ) containing an ideal…

2.ideal Gas - Final, PDF, Gases


Two closed bulbs of equal volume (V) containing an ideal gas initially pressure p and temperature T are connected through a narrow tube of negligible volume as shown in the figure below.

Two closed bulbs of equal volume V containing an ideal gas initially at pressure 1 atm and temperature 300 K are connected through a narrow tube of negligible volume as shown in

Consider the flasks in the following diagrams. Assuming the connecting tube has negligible volume, draw what each diagram will look like after the stopcock between the two flasks is opened. Also, solve
