Structure of the cross-β spine of amyloid-like fibrils
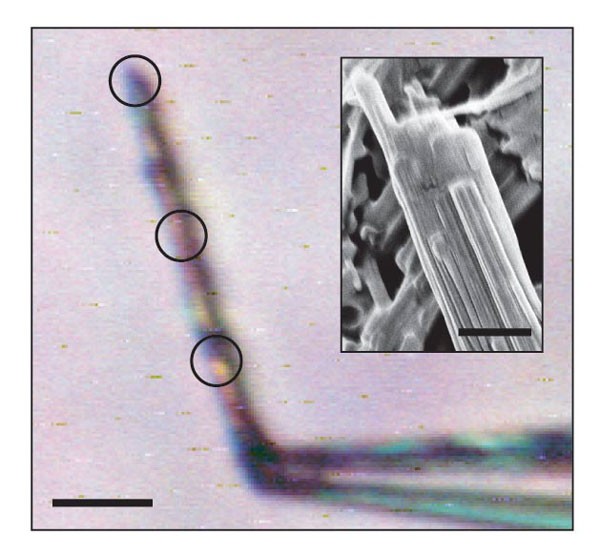

Amyloid - Wikipedia

Fibril formation from the amyloid-β peptide is governed by a dynamic equilibrium involving association and dissociation of the monomer. - Abstract - Europe PMC
Exploring the candidates for a new protein folding – cross-α amyloid – in available protein databases - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D0CP03256E

Dynamics of locking of peptides onto growing amyloid fibrils

PDF] Recent atomic models of amyloid fibril structure.

Atomic structures of amyloid cross-β spines reveal varied steric zippers

PDF] Recent atomic models of amyloid fibril structure.

RCSB PDB - 2ONV: Crystal Structure of the amyloid-fibril forming peptide GGVVIA derived from the Alzheimer's amyloid Abeta (Abeta37-42).
Thermodynamic Selection of Steric Zipper Patterns in the Amyloid Cross-β Spine

Elastic fibers and amyloid deposition in vascular tissue
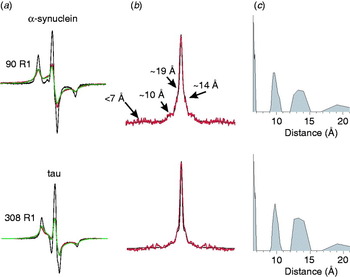
Fibrils with parallel in-register structure constitute a major class of amyloid fibrils: molecular insights from electron paramagnetic resonance spectroscopy, Quarterly Reviews of Biophysics

Cryo-EM of amyloid fibrils and cellular aggregates - ScienceDirect
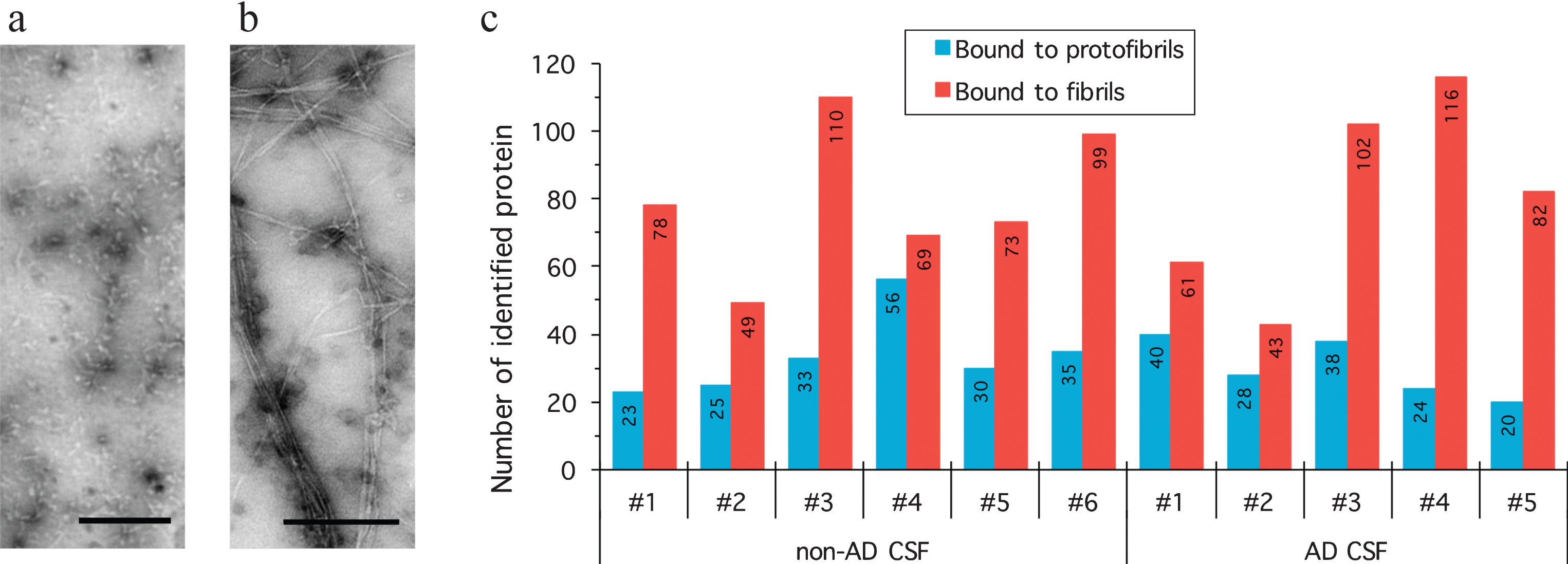
Protofibrillar and Fibrillar Amyloid-β Binding Proteins in Cerebrospinal Fluid - IOS Press