Applications for Medical Device Investigational Testing Authorizations Guidance Document
$ 22.50
In stock
4.9
(202)

Applications for Medical Device Investigational Testing Authorizations Guidance Document

US FDA Medical Device Applications

Current state of Health Canada regulation for cellular and gene

Health Canada released notice on research use only for COVID-19 tests
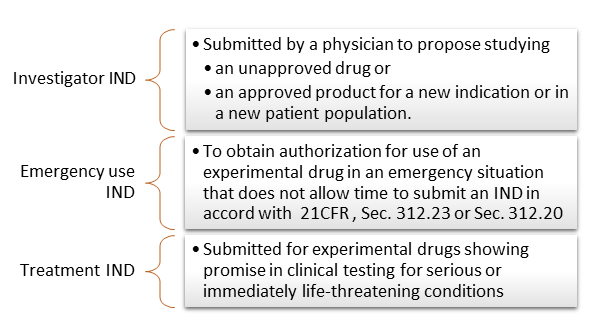
Investigational New Drug (IND) Application

FDA Emergency Use Authorizations
-image.jpg)
When does My Application Qualify for an Abbreviated 510(k)?
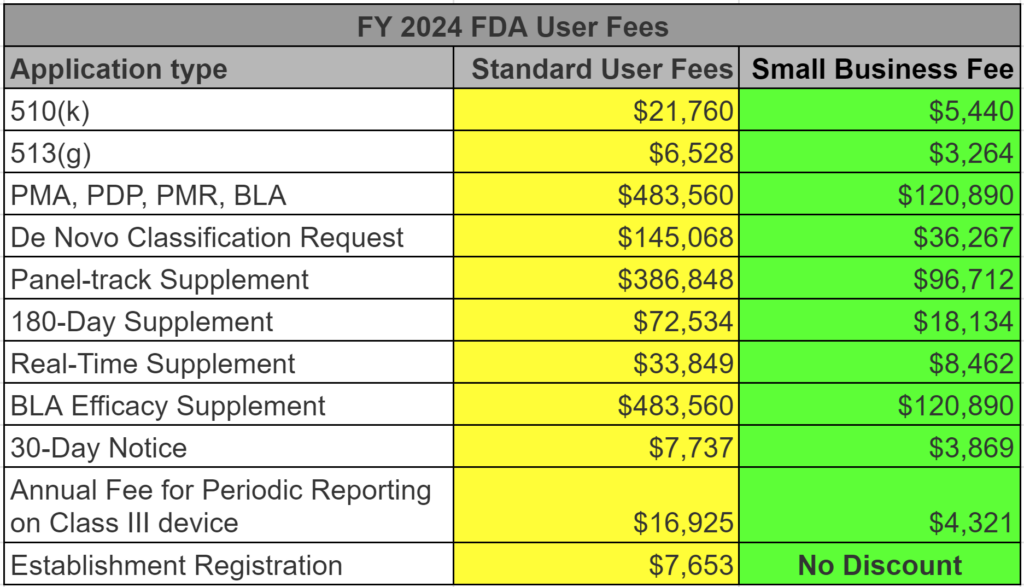
FDA

Applications for Medical Device Investigational Testing Authorizations Guidance Document

What Labs Need to Know About New Medical Device Cybersecurity Rules