At 300 K, 36 g of glucose present per litre in its solution has an osm

pi=CRT" (C = molar concentration)" (pi(1))/(pi(2))=(C(1))/(C(2))," "(4.98)/(1.52)=(36//180)/(C(2))" or "C(2)=(36)/(180)xx(1.52)/(4.98)="0.061 M"

Bioprocess engineering principles by Mauricio - Issuu

If the elevation in boiling point of a solution of 10 g of solute (mol
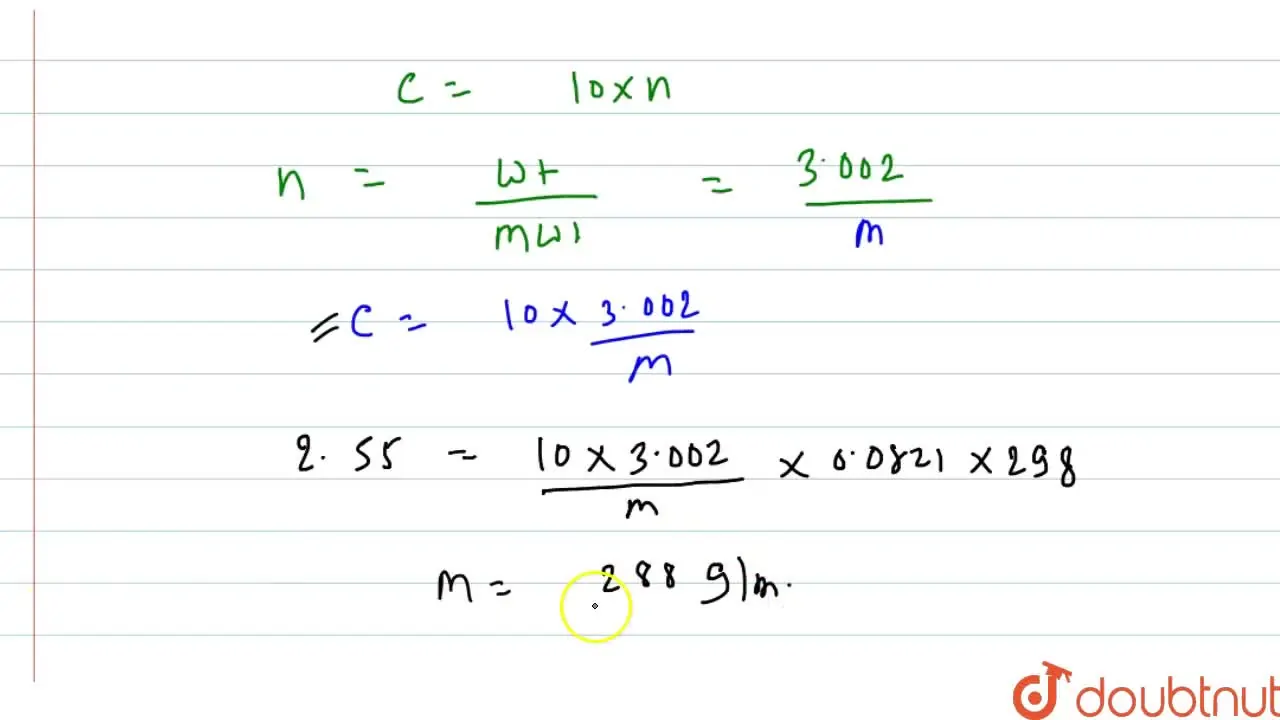
At 298 K, 100 cm^(3) of a solution containing 3.002 g of an unidentifi
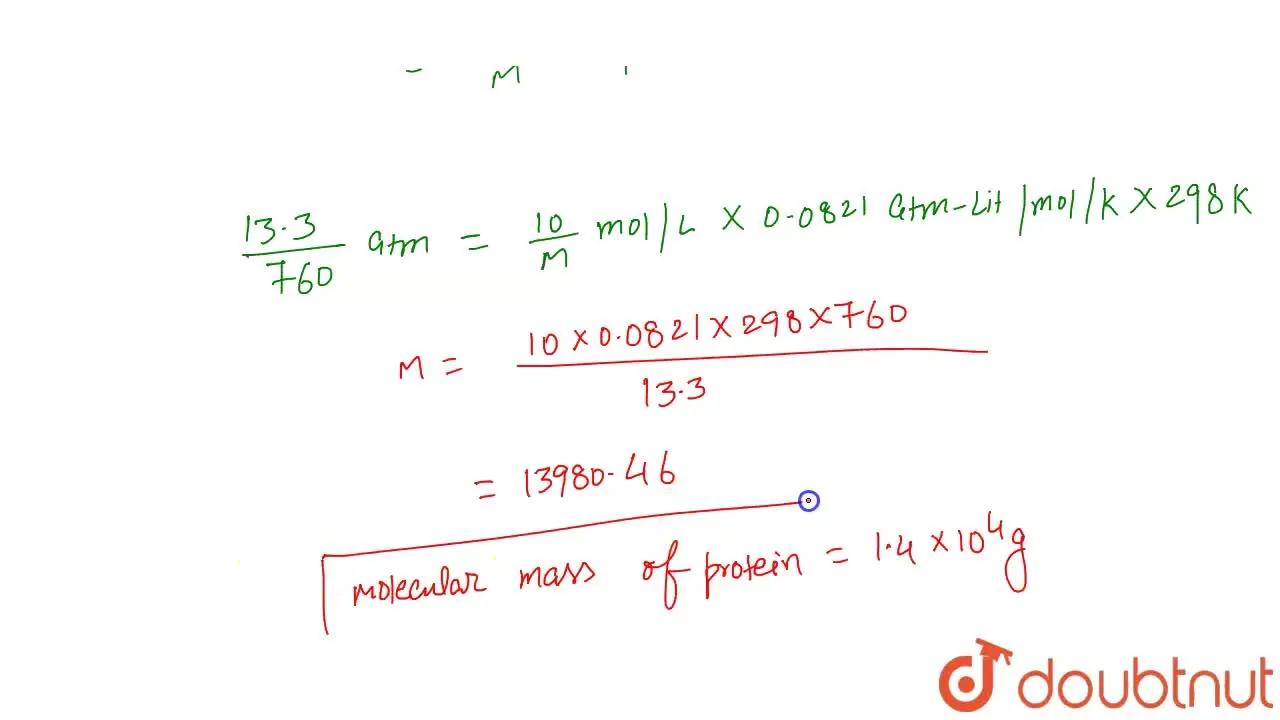
100 mg of a protein was disoved in just enough water to make 10 mL of

Solved] At 300 K,36 g of glucose present in a litre of its solution has ..

Solved] At 300 K,36 g of glucose present in a litre of its solution has ..

At 300 K,36 g of glucose present per litre in its solution has an osmotic..

PDF) Osmoles, osmolality and osmotic pressure: Clarifying the puzzle of solution concentration

Osmolarity, Definition, Units & Calculations - Lesson

Frontiers The Corrected Serum Sodium Concentration in Hyperglycemic Crises: Computation and Clinical Applications