117. Compressibility factor H, behaving as rea gas is 1) 1 RTV 3) 1+- RT 4) (1-a) 18. If V is the observed molor unlum

Click here:point_up_2:to get an answer to your question :writing_hand:117 compressibility factor for h behaving as reagas is1 1rtv31rt41a18 if v is the observed
Click here👆to get an answer to your question ✍️ 117- Compressibility factor H- behaving as rea gas is 1- 1 RTV 3- 1- RT 4- -1-a- 18- If V is the observed molor unlum

Explain how the compression factor varies with pressure and

Chemosensors, Free Full-Text
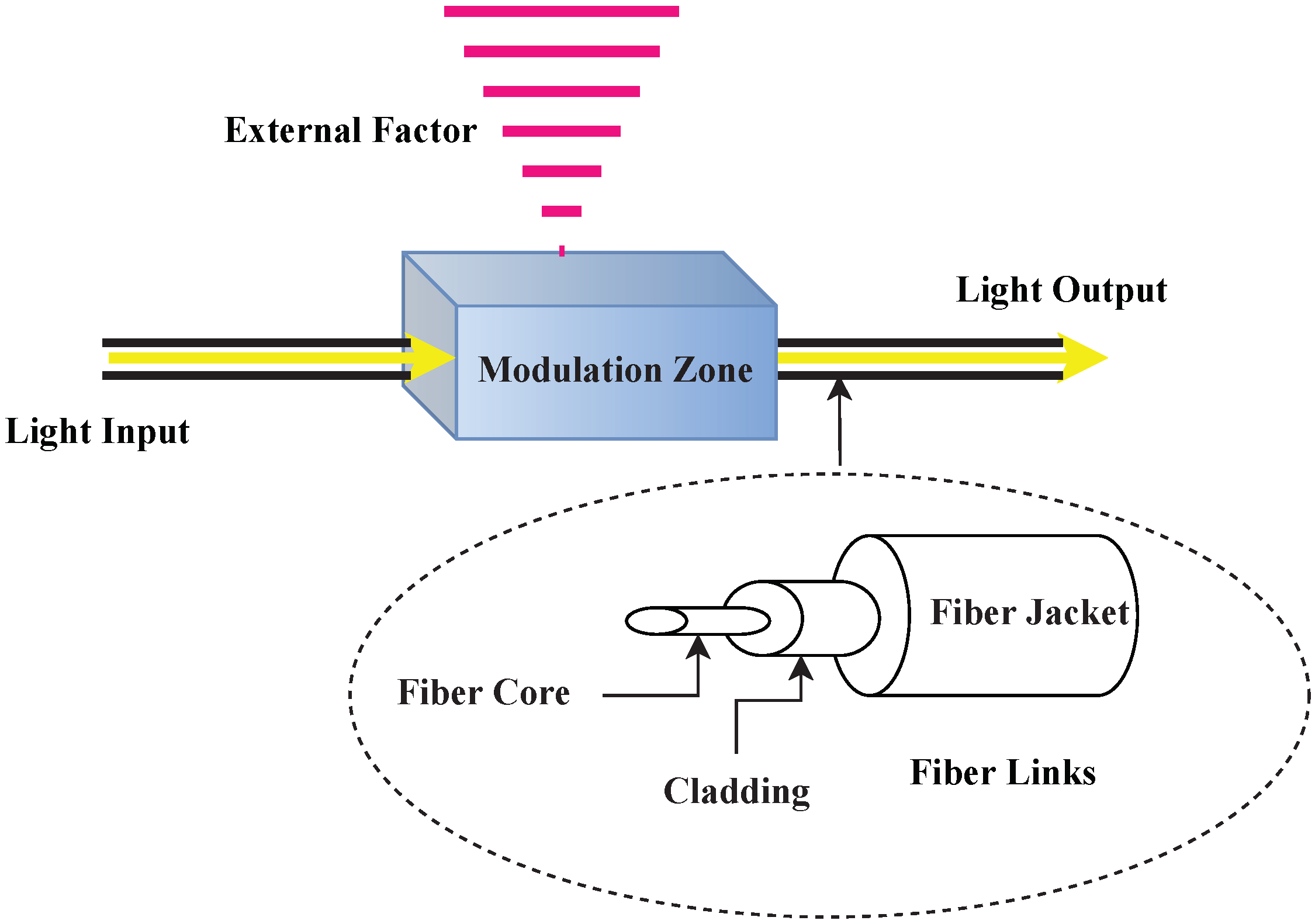
Sensors, Free Full-Text

Chemosensors, Free Full-Text

1 - Argonne National Laboratory
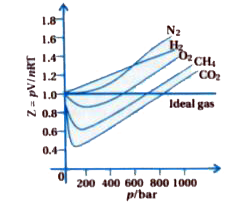
Gujrati] Explain compressibility factor (Z).

The compression factor (compressibility factor) for `1 mol` of a van der Waals gas at

Magnesium based materials for hydrogen based energy storage: Past, present and future - ScienceDirect

Carbon under pressure - ScienceDirect

8.6: Non-Ideal Gas Behavior General College Chemistry I