200 g of a sample of limestone liberates 66 g of CO2 on heating
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95
PDF) Chemical weathering and atmospheric carbon dioxide (CO2) consumption in Shanmuganadhi, South India: evidences from groundwater geochemistry
400 g sample of limestone liberates 44 g carbon dioxide on heating. The p..
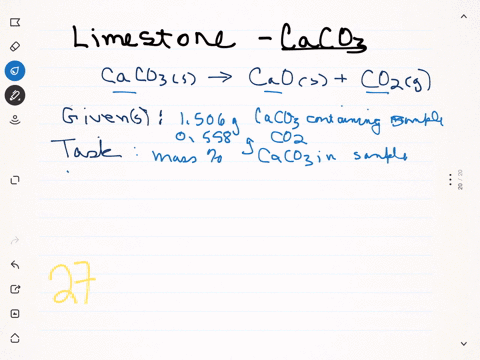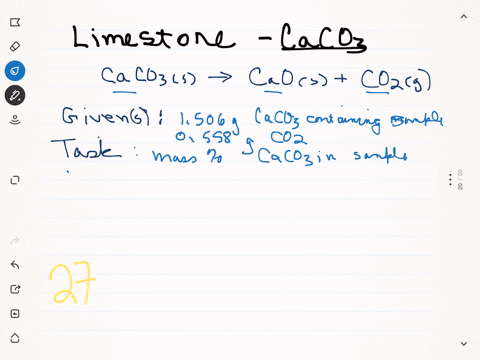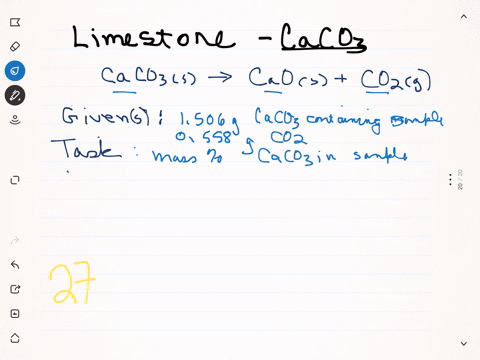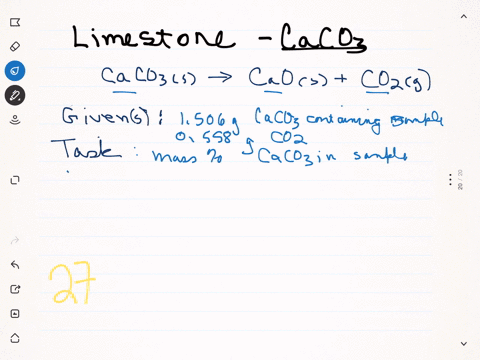
⏩SOLVED:A sample of limestone and other soil materials was heated

MC, PDF, Phase (Matter)
Cequest: Sequestering Carbon for Large-Scale Impact, by Naila Moloo
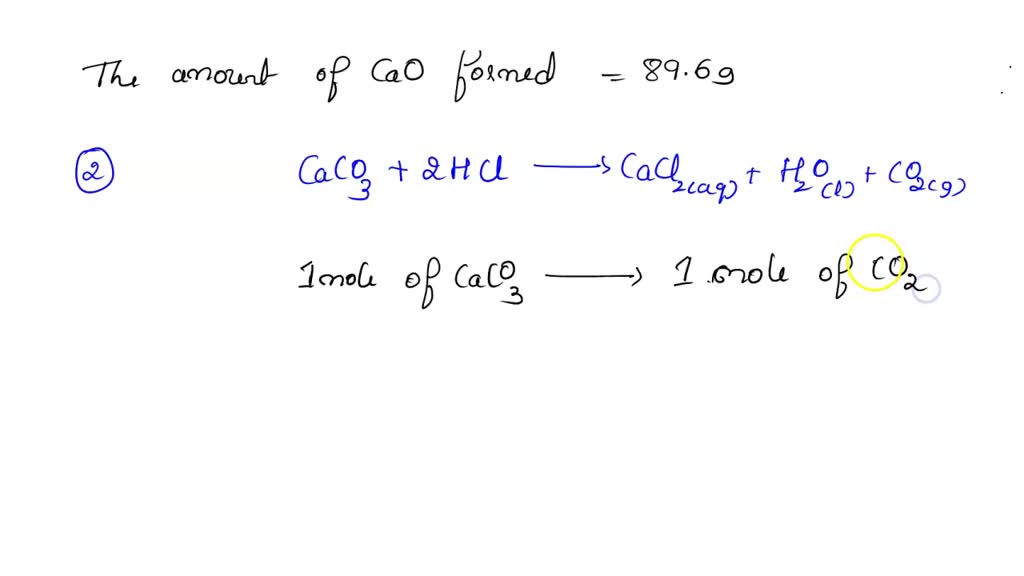
SOLVED: Calcium carbonate (limestone) decomposes when heated: CaCO3 CaO CO2 When 20.0g of calcium carbonate are decomposed, 11.2g of calcium oxide (lime), CaO, are formed. Calculate the mass of calcium oxide formed

US20060276339A1 - Methods and compositions for increasing the efficacy of biologically-active ingredients - Google Patents
Putting the Genie Back in the Toothpaste Tube
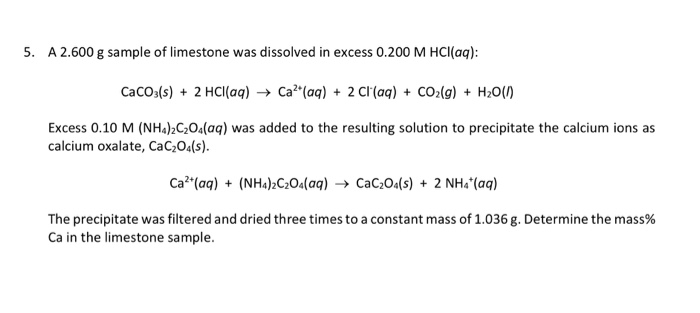
Solved A 2.600 g sample of limestone was dissolved in excess

Engineering Chemistry - Webs

A review on chemical precipitation in carbon capture, utilization

Calculate the enthalpy of the reaction 2NO(g) + O2(g) → 2NO2(g) g

Thermochemical energy storage system development utilising limestone - ScienceDirect

Techno-socio-economic aspects of Portland cement, Geopolymer, and Limestone Calcined Clay Cement (LC3) composite systems: A-State-of-Art-Review - ScienceDirect