SOLVED: A 25 gram piece of hot metal at 97°C is plunged into a 35
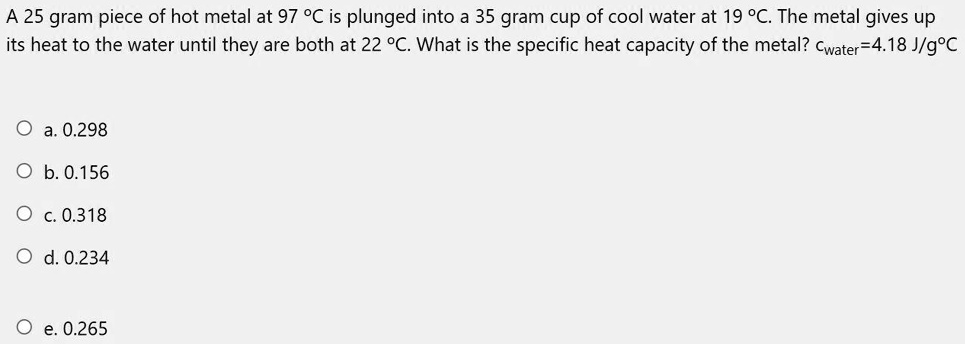

Solved A 25.0 g sample of a metal at 87.7DegC is placed in

SOLVED: A 22.1 g piece of aluminum (which has a molar heat

SOLVED: A 25.0 g piece of aluminum (which has a molar heat

SOLVED: A 12.9 gram sample of an unknown metal at 22.4°C is

Specific Heat Capacity

Solved 7. A hot metal with mass 16.33 grams is transferred

Solved Calculations Show the work for each step of the
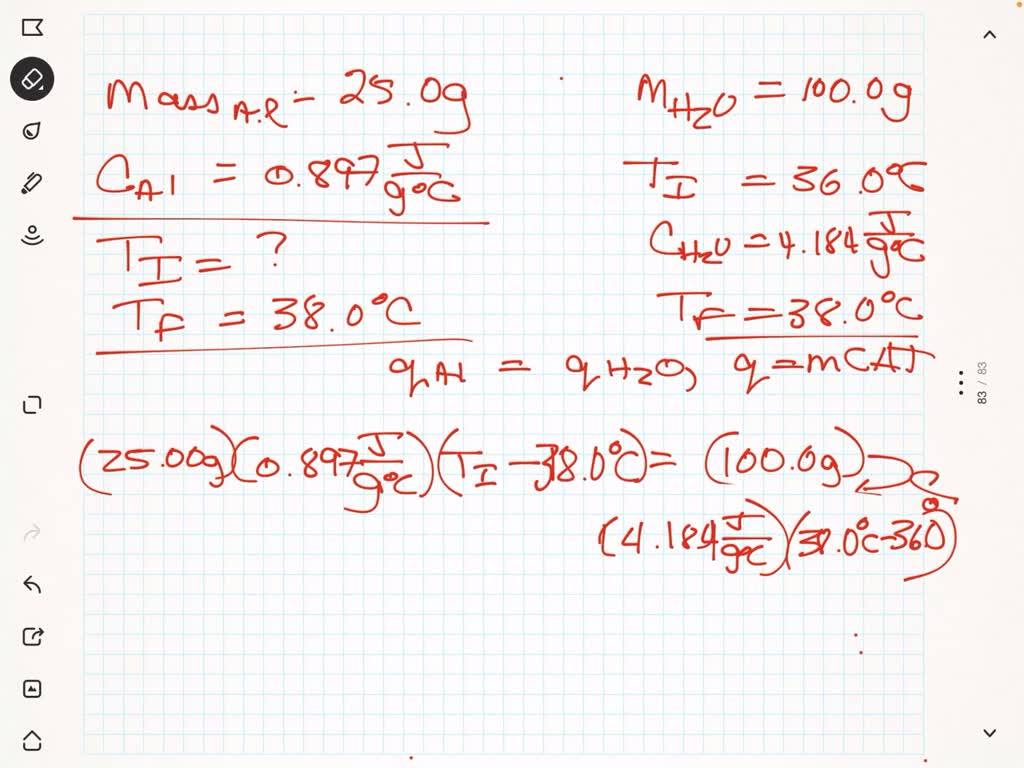
SOLVED: A 25.0 g block of Al with specific heat 0.897 J/g∘C is

Milwaukee 1-3/8 in. Carbide Universal Fit Extreme Wood and Metal
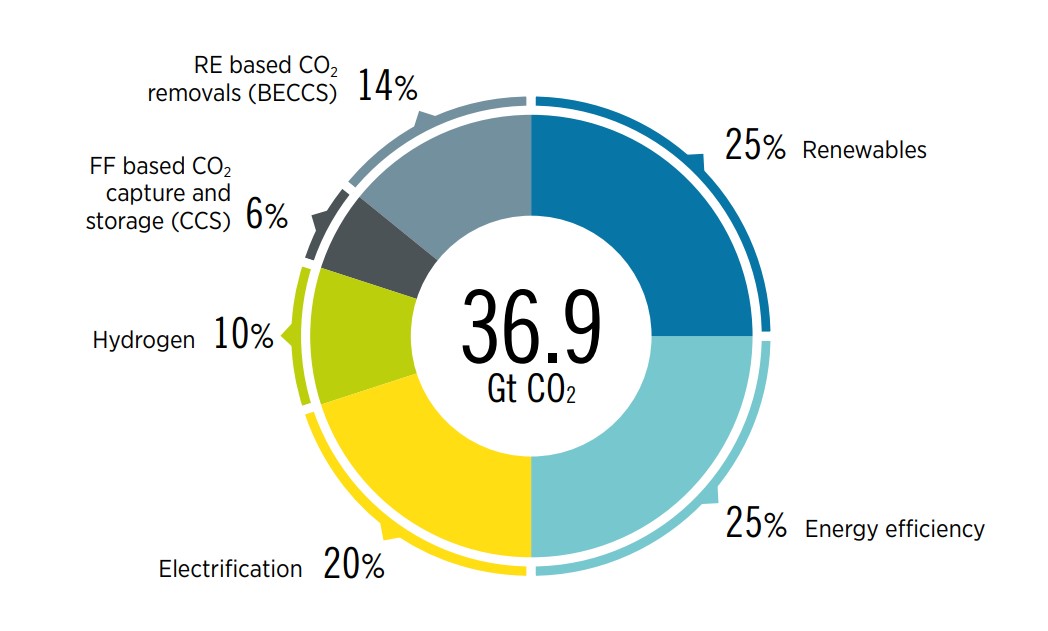
World Energy Transitions Outlook 2022
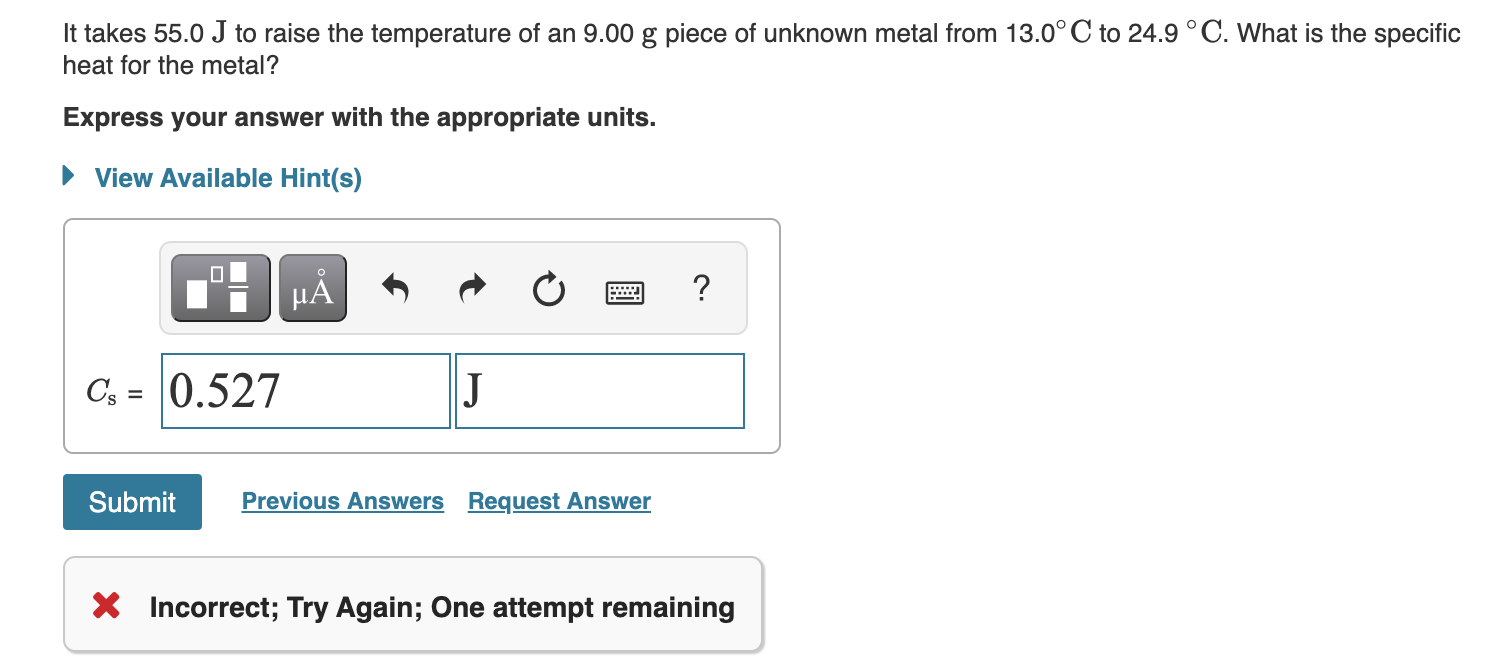
Solved The molar heat capacity of silver is 25.35 J/mol⋅∘C

Single-crystal structure determination of nanosized metal–organic