physical chemistry - Why do some gases have lower value of Z for a

In the above graph,the minima of the curve for methane is more than that of nitrogen. Also, for a given value of pressure, the value of $Z$ for methane is less than that of nitrogen. They seem to m
What is the significance of the curve part in Z vs. P graph of compressibility of a gas? - Quora

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Gas Compressibility - an overview

Critical Temperature - Temperature vs pressure graph, Examples

Ideal Gas Law Formula and Examples

Solid, Definition & Facts

Compressibility Factor Z & Real Gas Concept, States of Matter

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
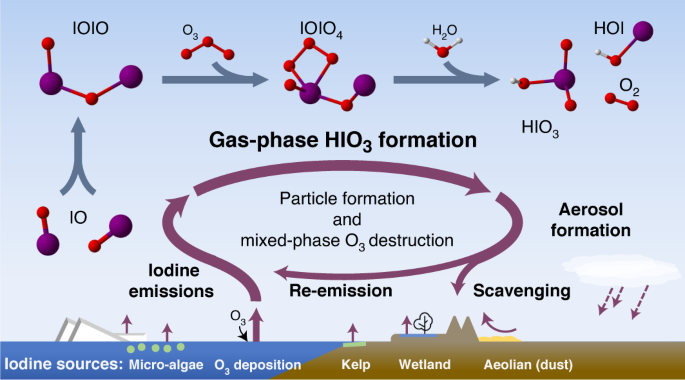
The gas-phase formation mechanism of iodic acid as an atmospheric aerosol source

Deviation Of Real Gas From Ideal Gas Behavior

The Ideal Gas Law

Compressibility factor (z): real gases deviate from ideal behav-Turito

Gas Compressibility - an overview

Compressibility Factor Z Important Concepts and Tips for JEE Main