PDF] Hemoglobin polymorphism in white-tailed deer: subunit basis

It was concluded from the results of limited structural studies that there were multiple peptide differences upon comparison of three non-α polypeptide chains in white-tailed deer. A variety of aberrant erythrocyte forms have been related to seven adult and two fetal hemoglobins in white-tailed deer. While sickling of the erythrocyte was not associated with a single hemoglobin type, it was precluded by hemoglobin V or VII, even when in combination with other hemoglobin types normally associated with sickling. The subunit basis of the hemoglobin polymorphism was presented. Two kinds of α subunits, six kinds of β subunits and one γ subunit were related to the whole hemoglobin molecule. The heterogeneity of the deer hemoglobins was based upon a variety of combinations of these numerous polypeptide chains. It was concluded from the results of limited structural studies that there were multiple peptide differences upon comparison of three non-α polypeptide chains.

APPENDIX B-Comparative Hematology, PDF, White Blood Cell

The conserved Phe GH5 of importance for hemoglobin intersubunit

Relaxed functional constraints on triplicate α-globin gene in the

PDF] Hemoglobin polymorphism in white-tailed deer: subunit basis

Genomic insights into the Ixodes scapularis tick vector of Lyme
Alteration of the α1β2/α2β1 subunit interface contributes to the

Physiologic and Genetic Factors Influencing the Zoonotic Cycle of

A Structural Analysis of the FDA Green Book-Approved Veterinary

Animals, Free Full-Text

PDF) Development of a host blood meal database: De novo sequencing

Ultrastructure of Sickled Deer Erythrocytes. I. The Typical
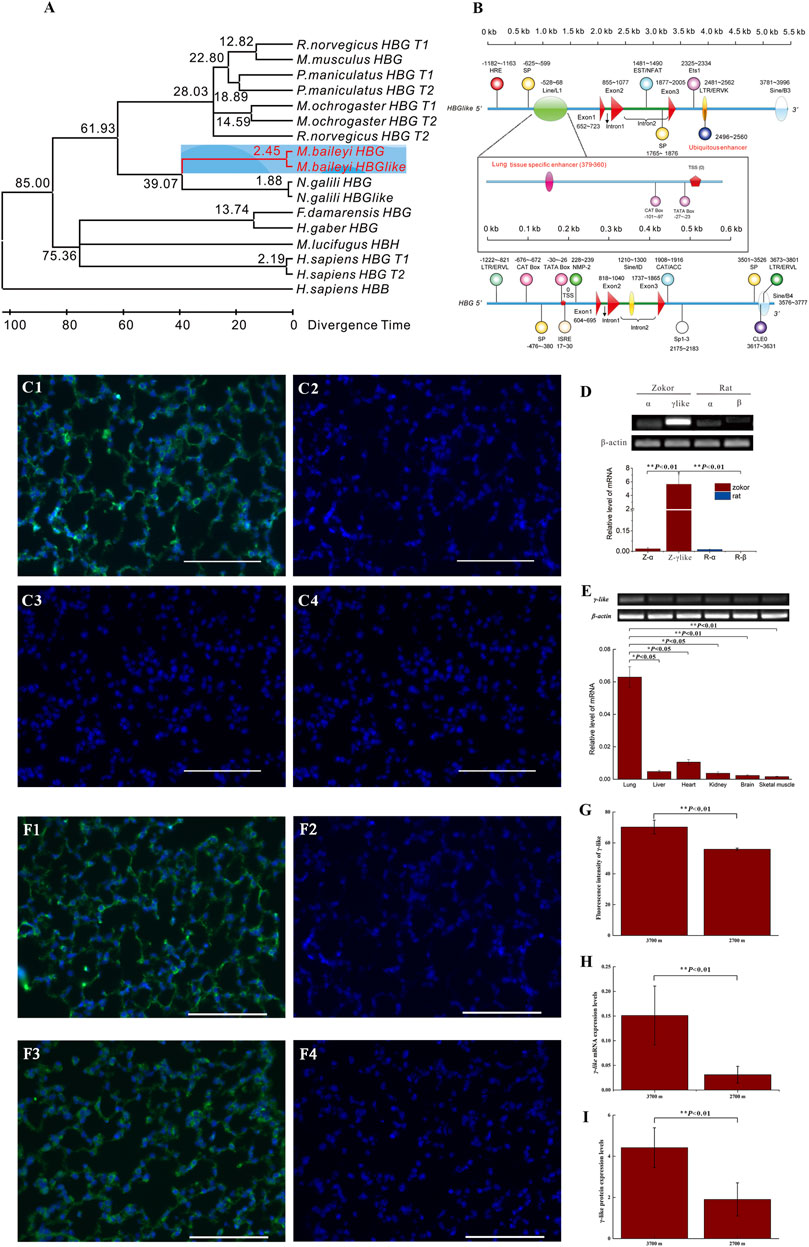
Frontiers A New Homotetramer Hemoglobin in the Pulmonary
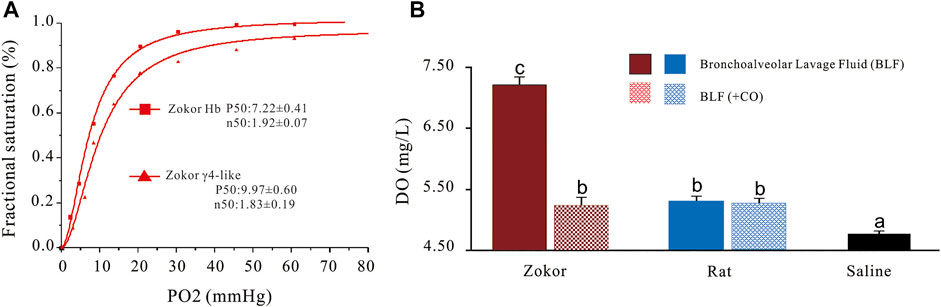
Frontiers A New Homotetramer Hemoglobin in the Pulmonary

High-altitude deer mouse hypoxia-inducible factor-2α shows