32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent

Ethylene oxide - Wikipedia

Visualizing Limiting Reactant - ppt download

SOLVED: Question 1: CH4 + 2 H2O → 4 H2 + CO2 Given 80 g of CH4 and 16.3 g of water, what is the limiting reactant?

How many grams of water are produced if we react 3 moles of hydrogen with 3 moles of oxygen? About 60 grams

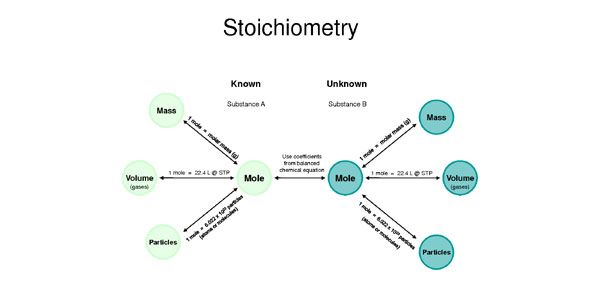
Stoichiometry And Limiting Reagent Review - Quiz, Trivia & Questions

Materials, Free Full-Text

Interface, Vol. 32, No. 2, Summer 2023 by The Electrochemical Society - Issuu

52. 80 g of H, is reacted with 80 g of O, to form water. Find out the mass of water obtained. Which substance is the limiting reagent?