SOLVED: 1.Convert 35C to F 2.Determine the amount of heat needed to raise the temperature of 200g of water from 0C to 67C.The specific heat 1 cal/(g-C 3.Determine the heat needed to
VIDEO ANSWER: Let's take a look at this question. In this question, the pressure will be the same as it was in the question. F1 by A1 is equal to F2 by A2 so we can write it that…
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

Calculate the time required to heat 20 kg of water from `10^(@)C` to `35^(@)C` using an imm
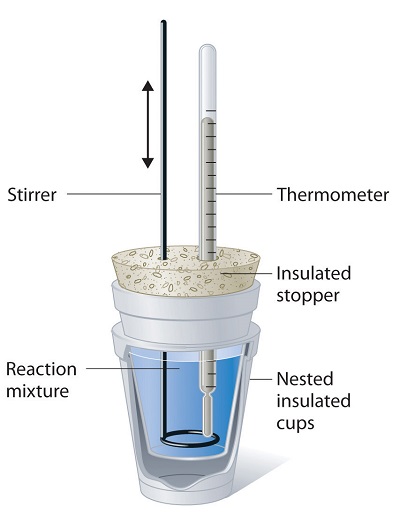
12.3: Heat Capacity, Enthalpy, and Calorimetry - Chemistry LibreTexts


Final Temperature of Ice and Water Mixture - How Many Grams of Ice Will Melt?

Complex Calorimetry Notes
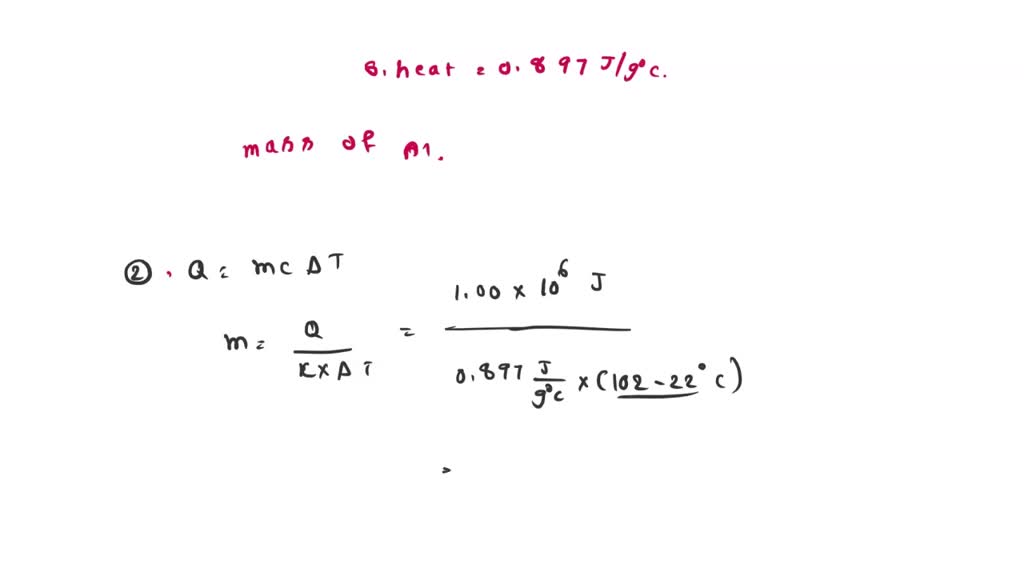
SOLVED: 5.2-1 Heat Calculations 1. Calculate the quantity of thermal energy required to warm 1.25 L of water from 22.0 °C to 98.0 °C in an electric kettle. The specific heat capacity

Specific Heat Capacity

Solved Solve Please. This is from another question an
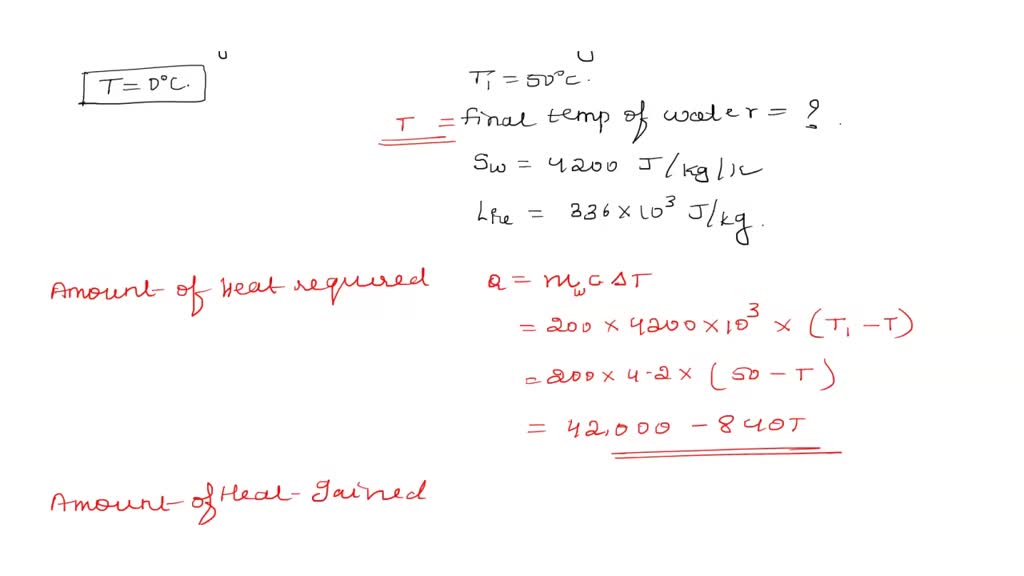
SOLVED: A piece of ice of mass 40 g is added to 200 g of water at 50oC. Calculate the final temperature of water when all the ice has melted. Specific heat
How much energy is required to raise the temperature of 2kg of water from 25° Celsius to 35° Celsius? - Quora