What is the change in internal energy (in J) of a system that

I found an increase of 3100J Have a look
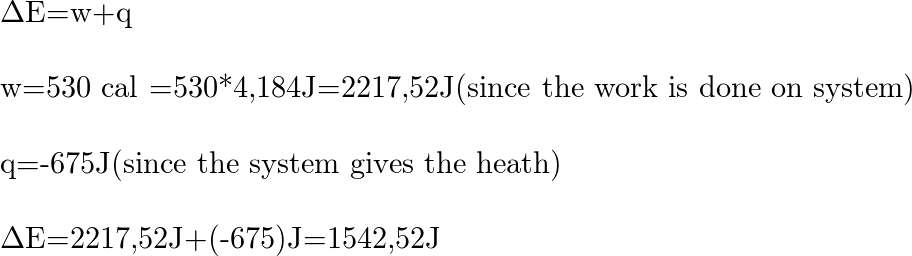
What is the change in internal energy (in J) of a system tha
If work done by the system is 300J when 100cal heat is supplied to it. Find the change in d internal energy during the process.
Solved] What is the change in internal energy (in J) of a system
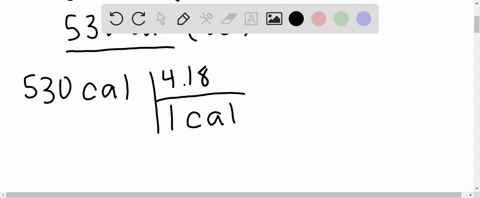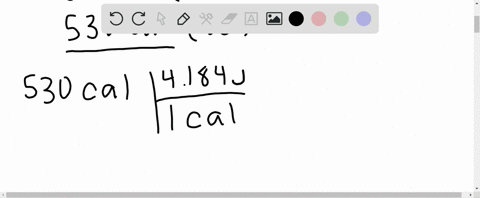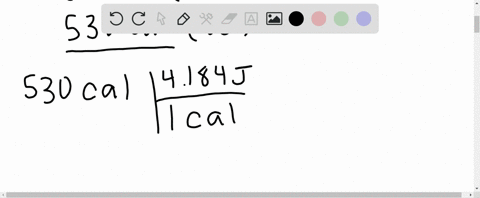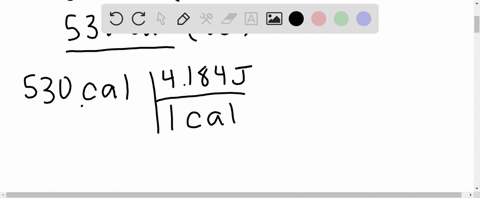
⏩SOLVED:What is the change in internal energy (in J) of a system

Answered: A certain amount of heat is transferred…

Ch6.1 The Nature of Energy (hustle!) - ppt download

What is the change in internal energy ΔU, for a system that does 70 J of ..

If change in internal energy is given by 120 J. The work done by the system is 280 J. Calculate the heat associated with the process.A. 160 JB. 160 JC. 400 JD. 400 J

OpenStax College Physics, Chapter 15, Problem 4 (Problems & Exercises)