24. Assertion :In B2H6, the terminal B H bonds are shorter, than
24. Assertion :In B2H6, the terminal B H bonds are shorter, than the B H bridge bonds Reason: The terminal B H bond order is greater than that of the B H bridge bond
24- Assertion-In B2H6- the terminal B-H bonds are shorter- than the B-H bridge bonds Reason- The terminal B-H bond order is greater than that of the B-H bridge bond
24. Assertion :In B2H6, the terminal B H bonds are shorter, than the B H bridge bonds Reason: The terminal B H bond order is greater than that of the B H bridge bond

Why is bridge bond stronger but longer in diborane? - Quora

Three-center two-electron bonds in the boranes B2H6 and B3H8− from the quantum interference perspective

Chemsiry Solutions, PDF, Mole (Unit)

Identify correct order of bond angles (A) C120 > F20 and F20 AsBrz > AsCl3 (C) NO > NOZ vdrogen of B2H6 and Hy is the bridging (D) HBH, >H.BH,; where H

Mechanistic Investigation of Stoichiometric Alkyne Insertion into Pt−B Bonds and Related Chemistry Bearing on the Catalytic Diborylation of Alkynes Mediated by Platinum(II) Diboryl Complexes
How are the B-H-B bridge bonds formed in B2H6? - Quora
Why is diborane cleavage symmetrical and unsymmetrical? - Quora

In which of the following compound (s) terminal (2C - 2e^{-}) bond and bridge bonds are lying in same plane

The correct statements regarding diborane B 2 H 6 is/are:A. Maximum six hydrogen atoms can lie in a planeB. Maximum six atoms can lie in a planeC. Bridging B H b bond

NEET 2022 Question Paper, Answer Key by Experts - The Right Admission pvt. Limited by The Right Admission Private Limited - Issuu
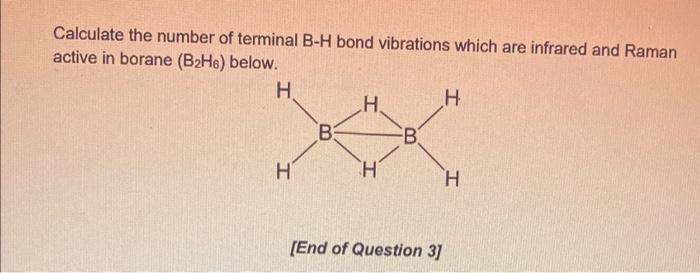
Solved Calculate the number of terminal B-H bond vibrations

NCERT Exemplar Class 11 Chemistry Solutions Chapter 11 - The P Block Elements

The Source Function Descriptor as a Tool to Extract Chemical Information from Theoretical and Experimental Electron Densities