20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20-If Z is a compressibility factor- van der Waals equation at low pressure can be written as
Van der Waals equation - Wikipedia

What is the compressibility factor (Z) for 0.02 mole of a van der Waal

SOLUTION: Hssrptr plus one che 5 states of matter question answers - Studypool

If Z is a compressibility factor, van der Waals equation low pressure can be written as: Z=1-displaystyle frac{Pb}{RT} Z=1+displaystyle frac{Pb}{RT} Z=1+displaystyle frac{RT}{Pb} Z=1-displaystyle frac{a}{VRT}

At low pressures For 1 mole, the van der Waals equation is written as [ p + a / V 2] V = RT The compressibility factor is then equal to:A. 1
The compressibility factor for one mol of a vanderwalls gas at 0 degree c and 100atm pressure is .5 then what will be the volume of 2 mols of this gas

At low pressure the van der Waals' equation is reduced to [P +(a)/(V^(

Real gases 1.4 Molecular interactions 1.5 The van de Waals equation 1.6 The principle of corresponding states Real gases do not obey the perfect gas law. - ppt download
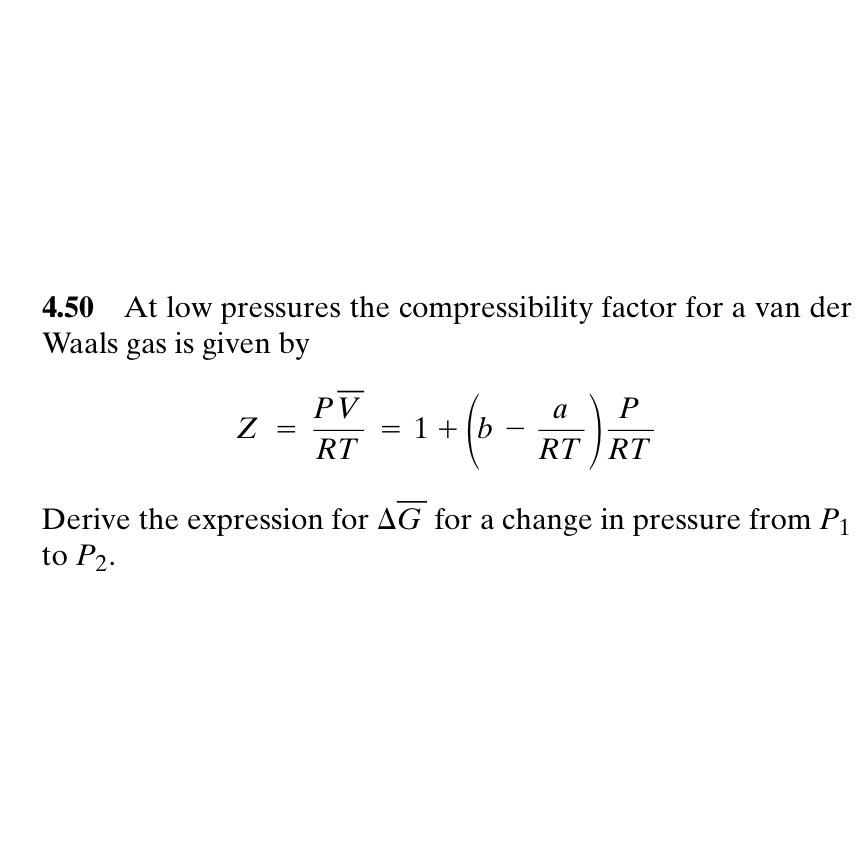
Solved 4.50 At low pressures the compressibility factor for

Solved) - For values of z near 1, it is a good approximation to write z(P) = - (1 Answer)