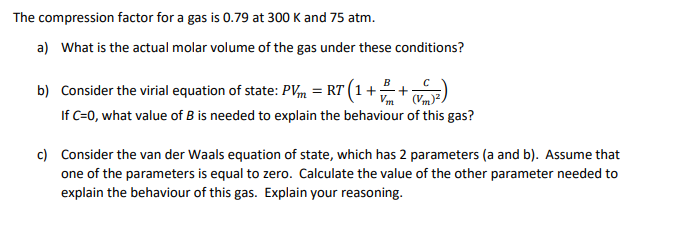
Solved The compression factor for a gas is 0.79 at 300 K and

The permeability of shale exposed to supercritical carbon dioxide

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0

Real Gas Behavior The Compression Factor (Z) [Example #2]

Solved The compression factor for a gas is 0.79 at 300 K and

Thermodynamics: Ideal Gas EOS and Compressibility Factor

Thermodynamics: Ideal Gas EOS and Compressibility Factor
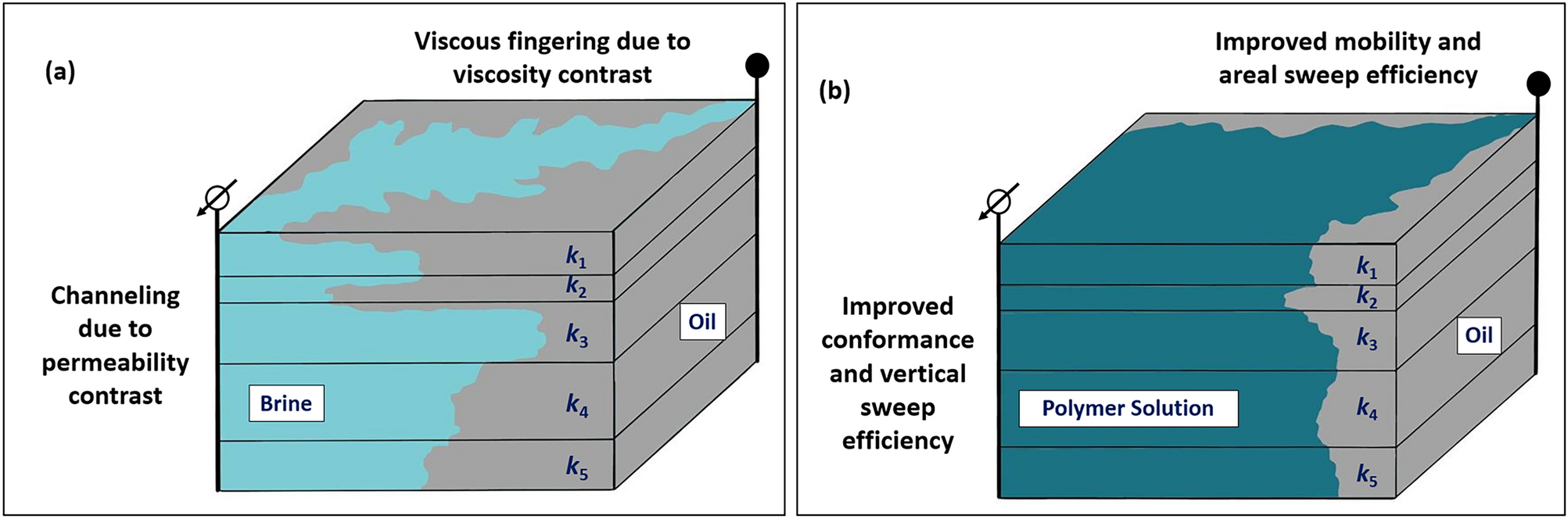
A comprehensive review of viscoelastic polymer flooding in sandstone and carbonate rocks
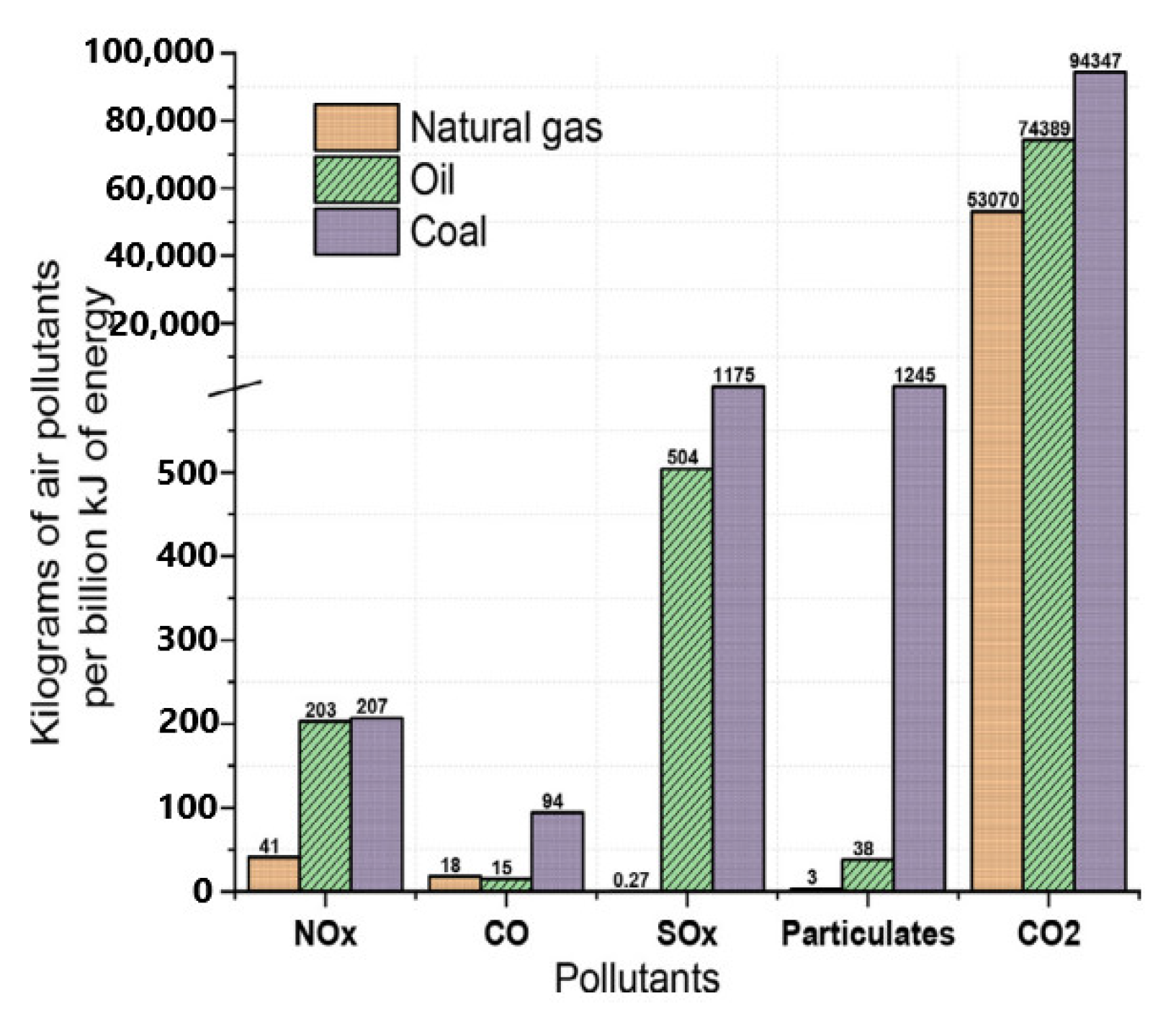
Energies, Free Full-Text

Calculate the compressibility factor for a gas, if 1 mole of it occupy 0.821 litre at 300 K and 50 atm.A. 1.33B. 1.67С. 0.67D. 1